LOSARTAN POTASSIUM Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Losartan Potassium, and when can generic versions of Losartan Potassium launch?
Losartan Potassium is a drug marketed by Aiping Pharm Inc, Alembic Pharms Ltd, Apotex Corp, Aurobindo Pharma, Chartwell Rx, Granules, Hetero Labs Ltd V, Hikma, Hisun Pharm Hangzhou, Ipca Labs Ltd, Jubilant Cadista, Lupin Ltd, Macleods Pharms Ltd, Micro Labs, MSN, Mylan, Prinston Inc, Strides Pharma, Teva, Torrent Pharms, Unichem, Watson Labs, Zydus Pharms Usa Inc, Apotex, Sandoz, and Teva Pharms. and is included in forty NDAs.
The generic ingredient in LOSARTAN POTASSIUM is hydrochlorothiazide; losartan potassium. There are thirty-two drug master file entries for this compound. Twenty-nine suppliers are listed for this compound. Additional details are available on the hydrochlorothiazide; losartan potassium profile page.
Summary for LOSARTAN POTASSIUM
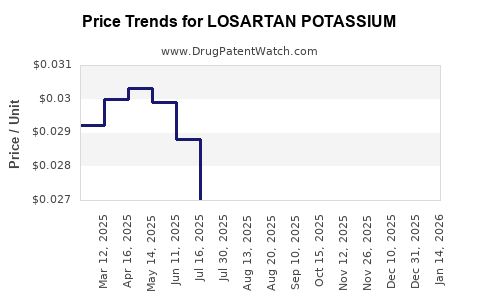
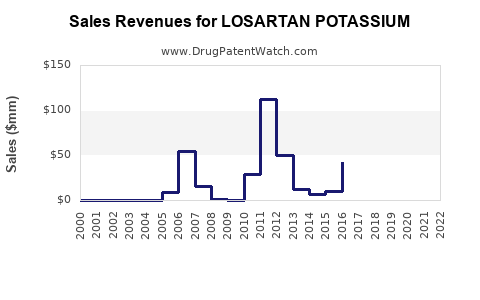
See drug prices for LOSARTAN POTASSIUM
Recent Clinical Trials for LOSARTAN POTASSIUM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Gadjah Mada University | Phase 1/Phase 2 |
| Second Affiliated Hospital, School of Medicine, Zhejiang University | N/A |
| Steadman Philippon Research Institute | Phase 2 |
Pharmacology for LOSARTAN POTASSIUM
| Drug Class | Angiotensin 2 Receptor Blocker |
| Mechanism of Action | Angiotensin 2 Receptor Antagonists |


