Exelixis Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for EXELIXIS INC, and when can generic versions of EXELIXIS INC drugs launch?
EXELIXIS INC has one approved drug.
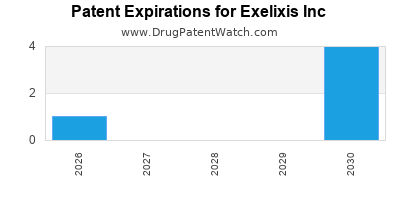
There are ten US patents protecting EXELIXIS INC drugs.
There are two hundred and seven patent family members on EXELIXIS INC drugs in thirty-two countries and nineteen supplementary protection certificates in sixteen countries.
Drugs and US Patents for Exelixis Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-001 | Apr 25, 2016 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-003 | Apr 25, 2016 | RX | Yes | Yes | 10,034,873 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-003 | Apr 25, 2016 | RX | Yes | Yes | 11,091,440 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-003 | Apr 25, 2016 | RX | Yes | Yes | 7,579,473 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-002 | Apr 25, 2016 | RX | Yes | No | 11,098,015 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-001 | Apr 25, 2016 | RX | Yes | No | 11,091,439 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Exelixis Inc | CABOMETYX | cabozantinib s-malate | TABLET;ORAL | 208692-001 | Apr 25, 2016 | RX | Yes | No | 9,724,342 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Exelixis Inc Drugs
Supplementary Protection Certificates for Exelixis Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2213661 | C02213661/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: CABOZANTINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66471 15.12.2017 |
| 2213661 | C20140029 00117 | Estonia | ⤷ Sign Up | PRODUCT NAME: KABOSANTINIIB;REG NO/DATE: K(2014)2043 (LOPLIK) 26.03.2014 |
| 2213661 | C 2014 036 | Romania | ⤷ Sign Up | PRODUCT NAME: CABOZANTINIB SI ORICE FORMA ECHIVALENTA TERAPEUTIC AACESTUIA, INCLUSIV SARURILE ACCEPT DATE OF NATIONAL AUTHORISATION: 20140321; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/890/001, EU/1/13/890/002, EU/1/13/890/003; DATE OF FIRST AUTHORISATION IN EEA: 20140321 ABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/13/890/001, EU/1/13/890/002, EU/1/13/890/003; |
| 2213661 | 300678 | Netherlands | ⤷ Sign Up | PRODUCT NAME: CABOZANTINIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/13/890/001-003 20140326 |
| 2213661 | CR 2014 00039 | Denmark | ⤷ Sign Up | PRODUCT NAME: CABOZANTINIB, INKLUSIVE FARMACEUTISK ACCEPTABLE SALTE HERAF, HERUNDER CABOZANTINIB (S)-MALAT; REG. NO/DATE: EU/1/13/890/001-003 20140326 |
| 2213661 | PA2014033,C2213661 | Lithuania | ⤷ Sign Up | PRODUCT NAME: KABOZANTINIBAS IR JO FARMACINIU POZIURIU PRIIMTINOS DRUSKOS; REGISTRATION NO/DATE: EU/1/13/890/001 - EU/1/13/890/003 20140321 |
| 2213661 | SPC/GB14/052 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: CABOZANTINIB AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU/1/13/890/001-006 20140326 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.