Dexamethasone - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for dexamethasone and what is the scope of freedom to operate?
Dexamethasone
is the generic ingredient in thirty-four branded drugs marketed by Allergan Herbert, Merck, Hikma, Alpharma Us Pharms, Anima, Chartwell Molecular, Lyne, Pharmobedient Cnsltg, Aspen Global Inc, Abbvie, Ocular Therapeutix, Watson Labs, Harrow Eye, Eyepoint Pharms, Alvogen, Amneal, Apotex, Bausch, Bionpharma, Chartwell Rx, Impax Labs, Larken Labs Inc, Novitium Pharma, Pangea, Phoenix Labs Ny, Prasco, Pvt Form, Roxane, Sun Pharm Industries, Upsher Smith, Whiteworth Town Plsn, Xspire Pharma, Zydus Pharms, Solvay, Dexcel, Watson Labs Teva, Ucb Inc, Cent Pharms, Abraxis Pharm, Fresenius Kabi Usa, Bel Mar, Dell Labs, Dr Reddys, Epic Pharma Llc, Eugia Pharma, Geneyork Pharms, Gland Pharma Ltd, Intl Medication, Luitpold, Lyphomed, Mylan Labs Ltd, Somerset, Somerset Theraps Llc, Teva Parenteral, West-ward Pharms Int, Wyeth Ayerst, Pharmafair, Alcon, Sola Barnes Hind, Bausch And Lomb, Sandoz, Alcon Pharms Ltd, Novartis, and Padagis Us, and is included in one hundred and forty-seven NDAs. There are fourteen patents protecting this compound. Additional information is available in the individual branded drug profile pages.Dexamethasone has seventy-one patent family members in twenty countries.
There are thirty-nine drug master file entries for dexamethasone. Forty-one suppliers are listed for this compound.
Summary for dexamethasone
| International Patents: | 71 |
| US Patents: | 14 |
| Tradenames: | 34 |
| Applicants: | 64 |
| NDAs: | 147 |
| Drug Master File Entries: | 39 |
| Finished Product Suppliers / Packagers: | 41 |
| Raw Ingredient (Bulk) Api Vendors: | 115 |
| Clinical Trials: | 3,400 |
| Patent Applications: | 7,332 |
| Formulation / Manufacturing: | see details |
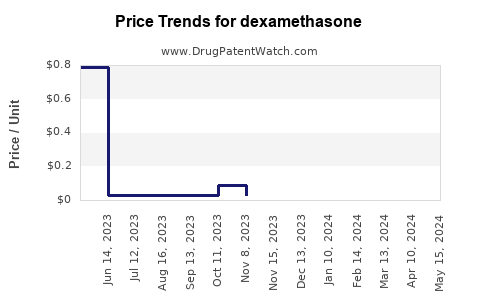
| Drug Prices: | Drug price trends for dexamethasone |
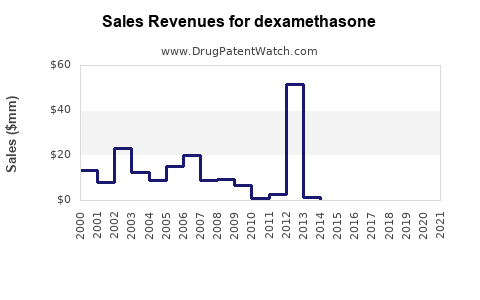
| Drug Sales Revenues: | Drug sales revenues for dexamethasone |
| What excipients (inactive ingredients) are in dexamethasone? | dexamethasone excipients list |
| DailyMed Link: | dexamethasone at DailyMed |
Recent Clinical Trials for dexamethasone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dipenkumar Modi | Phase 2 |
| National Trauma Center | N/A |
| Genmab | Phase 2 |
Pharmacology for dexamethasone
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Medical Subject Heading (MeSH) Categories for dexamethasone
Anatomical Therapeutic Chemical (ATC) Classes for dexamethasone
US Patents and Regulatory Information for dexamethasone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xspire Pharma | DEXAMETHASONE | dexamethasone | TABLET;ORAL | 088237-001 | Apr 28, 1983 | BP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Alvogen | DEXAMETHASONE | dexamethasone | TABLET;ORAL | 088481-003 | Apr 28, 1983 | BP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Epic Pharma Llc | DEXAMETHASONE SODIUM PHOSPHATE | dexamethasone sodium phosphate | SOLUTION/DROPS;OTIC | 084855-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ucb Inc | DEXACORT | dexamethasone sodium phosphate | AEROSOL;NASAL | 014242-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Aspen Global Inc | HEXADROL | dexamethasone sodium phosphate | INJECTABLE;INJECTION | 014694-002 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Larken Labs Inc | DEXAMETHASONE | dexamethasone | TABLET;ORAL | 201270-001 | Jul 17, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for dexamethasone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | OZURDEX | dexamethasone | IMPLANT;INTRAVITREAL | 022315-001 | Jun 17, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for dexamethasone
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AbbVie Deutschland GmbH & Co. KG | Ozurdex | dexamethasone | EMEA/H/C/001140 Ozurdex is indicated for the treatment of adult patients with macular oedema following either branch retinal-vein occlusion (BRVO) or central retinal-vein occlusion (CRVO).Ozurdex is indicated for the treatment of adult patients with inflammation of the posterior segment of the eye presenting as noninfectious uveitis.Ozurdex is indicated for the treatment of adult patients with visual impairment due to diabetic macular oedema (DME) who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for non-corticosteroid therapy. |
Authorised | no | no | no | 2010-07-26 | |
| THERAVIA | Neofordex | dexamethasone | EMEA/H/C/004071 Treatment of multiple myeloma. |
Authorised | no | no | no | 2016-03-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for dexamethasone
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2017062770 | ⤷ Try a Trial | |
| Brazil | 112013027428 | guias de dose para seringa de injeção | ⤷ Try a Trial |
| China | 110339153 | 地塞米松单位剂型、试剂盒及白内障手术后炎症中的应用 (Use of sustained release dexamethasone in post-cataract surgery inflammation) | ⤷ Try a Trial |
| South Korea | 20190042765 | 주사기용 투여량 가이드 (DOSE GUIDES FOR INJECTION SYRINGE) | ⤷ Try a Trial |
| Australia | 2010213612 | Drug delivery through hydrogel plugs | ⤷ Try a Trial |
| Australia | 2010246115 | Biomaterials for track and puncture closure | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for dexamethasone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1581193 | SPC/GB12/047 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DEXAMETHASONE; REGISTERED: UK EU/1/10/638/001 20100727 |
| 1429780 | SPC/GB12/058 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF CIPROFLOXACIN AND DEXAMETHASONE, PREFERABLY CIPROFLOXACIN HYDROCHLORIDE AND DEXAMETHASONE; REGISTERED: DK DE/11/3337/001/DC 20120808; UK PL000649/0381-0001 20121003 |
| 1429780 | 13C0012 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE CIPROFLOXACINE ET DE DEXAMETHASONE, EN PARTICULIER DE CHLORHYDRATE DE CIPROFLOXACINE ET DE DEXAMETHASONE; NAT. REGISTRATION NO/DATE: NL 41308 20121214; FIRST REGISTRATION: 48976 20120808 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.