Novartis Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVARTIS, and when can generic versions of NOVARTIS drugs launch?
NOVARTIS has one hundred and eighty-five approved drugs.
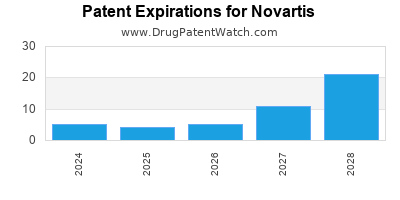
There are one hundred and three US patents protecting NOVARTIS drugs.
There are two thousand seven hundred and forty-seven patent family members on NOVARTIS drugs in sixty-nine countries and five hundred and twenty supplementary protection certificates in nineteen countries.
Summary for Novartis
| International Patents: | 2747 |
| US Patents: | 103 |
| Tradenames: | 154 |
| Ingredients: | 139 |
| NDAs: | 185 |
| Drug Master File Entries: | 1 |
| Patent Litigation for Novartis: | See patent lawsuits for Novartis |
| PTAB Cases with Novartis as patent owner: | See PTAB cases with Novartis as patent owner |
Drugs and US Patents for Novartis
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-003 | Aug 24, 2021 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | RX | Yes | Yes | 9,309,229 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Novartis Pharms Corp | JADENU | deferasirox | TABLET;ORAL | 206910-003 | Mar 30, 2015 | AB | RX | Yes | Yes | 9,283,209 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Novartis Pharms Corp | ENTRESTO | sacubitril; valsartan | TABLET;ORAL | 207620-001 | Jul 7, 2015 | RX | Yes | No | 11,058,667 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | APRESAZIDE | hydralazine hydrochloride; hydrochlorothiazide | CAPSULE;ORAL | 084735-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Novartis | KISQALI FEMARA CO-PACK (COPACKAGED) | letrozole; ribociclib succinate | TABLET;ORAL | 209935-001 | May 4, 2017 | RX | Yes | Yes | 9,416,136 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Novartis
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | STARLIX | nateglinide | TABLET;ORAL | 021204-002 | Dec 22, 2000 | 6,641,841 | ⤷ Try a Trial |
| Novartis | AMTURNIDE | aliskiren hemifumarate; amlodipine besylate; hydrochlorothiazide | TABLET;ORAL | 200045-002 | Dec 21, 2010 | 5,559,111 | ⤷ Try a Trial |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-002 | Nov 20, 2008 | 7,332,481*PED | ⤷ Try a Trial |
| Novartis | ESTRADERM | estradiol | SYSTEM;TRANSDERMAL | 019081-002 | Sep 10, 1986 | 4,379,454 | ⤷ Try a Trial |
| Novartis | LIORESAL | baclofen | TABLET;ORAL | 017851-003 | Jan 20, 1982 | 3,471,548 | ⤷ Try a Trial |
| Novartis | NEORAL | cyclosporine | CAPSULE;ORAL | 050715-003 | Jul 14, 1995 | 5,342,625 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVARTIS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 5 mg/160 mg | ➤ Subscribe | 2007-10-22 |
| ➤ Subscribe | Capsules | 150 mg and 200 mg | ➤ Subscribe | 2013-01-29 |
| ➤ Subscribe | Tablets | 10 mg/160 mg | ➤ Subscribe | 2007-10-01 |
| ➤ Subscribe | Injection | 4 mg/100 mg, 100 mL vial | ➤ Subscribe | 2012-01-31 |
| ➤ Subscribe | Tablets | 5 mg/12.5 mg/160 mg, 5 mg/25 mg/160 mg, 10 mg/25 mg/160 mg and 10 mg/25 mg/320 m | ➤ Subscribe | 2009-09-14 |
| ➤ Subscribe | Nasal Spray | 0.665 mg/ Spray | ➤ Subscribe | 2009-06-29 |
| ➤ Subscribe | Tablets | 125 mg, 250 mg and 500 mg | ➤ Subscribe | 2004-12-28 |
| ➤ Subscribe | Oral Suspension | 300 mg/5 mL | ➤ Subscribe | 2006-12-26 |
| ➤ Subscribe | Tablets | 12.5 mg and 25 mg | ➤ Subscribe | 2014-02-04 |
| ➤ Subscribe | Delayed-release Tablets | 180 mg | ➤ Subscribe | 2009-06-04 |
| ➤ Subscribe | Tablets | 250 mg | ➤ Subscribe | 2011-03-14 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Tablets | 125 mg, 250 mg, and 500 mg | ➤ Subscribe | 2011-10-28 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Tablets for Oral Suspension | 2 mg, 3 mg and 5 mg | ➤ Subscribe | 2016-12-30 |
| ➤ Subscribe | Tablets | 2.5 mg, 5 mg, and 7.5 mg | ➤ Subscribe | 2014-12-10 |
| ➤ Subscribe | Tablets | 100 mg and 400 mg | ➤ Subscribe | 2007-03-12 |
| ➤ Subscribe | Tablets | 90 mg and 360 mg | ➤ Subscribe | 2015-10-19 |
| ➤ Subscribe | Tablets | 320 mg/12.5 mg and 320 mg/25 mg | ➤ Subscribe | 2007-02-07 |
| ➤ Subscribe | Capsules | 20 mg and 40 mg | ➤ Subscribe | 2008-06-04 |
| ➤ Subscribe | Tablets | 180 mg | ➤ Subscribe | 2016-04-28 |
| ➤ Subscribe | Tablets | 5 mg/320 mg | ➤ Subscribe | 2007-11-26 |
| ➤ Subscribe | Injection | 0.8 mg (base) /mL | ➤ Subscribe | 2008-06-11 |
| ➤ Subscribe | Tablets | 10 mg/320 mg | ➤ Subscribe | 2007-11-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.003% | ➤ Subscribe | 2015-12-30 |
| ➤ Subscribe | Tablets | 10 mg/12.5 mg/160 mg | ➤ Subscribe | 2009-10-22 |
| ➤ Subscribe | Tablets | 150 mg, 300 mg and 600 mg | ➤ Subscribe | 2006-05-05 |
| ➤ Subscribe | Tablets | 50 mg and 75 mg | ➤ Subscribe | 2014-01-07 |
| ➤ Subscribe | Delayed-release Tablets | 360 mg | ➤ Subscribe | 2009-02-02 |
| ➤ Subscribe | Tablets | 60 mg and 120 mg | ➤ Subscribe | 2004-12-22 |
| ➤ Subscribe | Tablets | 40 mg, 80 mg,160 mg | ➤ Subscribe | 2004-12-28 |
| ➤ Subscribe | Extended-release Tablets | 100 mg | ➤ Subscribe | 2005-12-30 |
| ➤ Subscribe | Tablets | 0.25 mg, 0.5 mg, and 0.75 mg | ➤ Subscribe | 2013-09-30 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
| ➤ Subscribe | Tablets | 10 mg | ➤ Subscribe | 2014-06-18 |
| ➤ Subscribe | Capsules | 0.5 mg | ➤ Subscribe | 2014-09-22 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2006-03-02 |
| ➤ Subscribe | Tablets | 80 mg/12.5 mg, 160 mg/12.5 mg and 160 mg/25 mg | ➤ Subscribe | 2005-12-02 |
| ➤ Subscribe | Capsules | 400 mg | ➤ Subscribe | 2014-01-24 |
| ➤ Subscribe | Tablets | 180 mg | ➤ Subscribe | 2015-10-23 |
International Patents for Novartis Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| San Marino | P200800069 | ⤷ Try a Trial |
| China | 1615134 | ⤷ Try a Trial |
| European Patent Office | 2021507 | ⤷ Try a Trial |
| European Patent Office | 2152237 | ⤷ Try a Trial |
| Japan | 7407508 | ⤷ Try a Trial |
| Hong Kong | 1197723 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Novartis Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2340828 | LUC00195 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL ET VALSARTAN, SOUS FORME DE COMPLEXE DE SEL DE SODIUM SACUBITRIL VALSARTAN, C'EST-A-DIRE (((S)-N-VALERYL-N-((2'-(1H-TETRAZOLE-5-YL)-BIPHENYL-4-YL)-METHYL)-VALINE) ((2R,4S)-5-BIPHENYL-4-YL-4-(3-CARBOXY-PROPIONYLAMINO)-2-METHYL-ESTER ETHYLIQUE D'ACIDE PENTANOIQUE))NA3 X H2O, DANS LEQUEL X EST 0 A 3; AUTHORISATION NUMBER AND DATE: EU/1/15/1058 20151123 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 2379069 | LUC00160 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; AUTHORISATION NUMBER AND DATE: EU/1/19/1414 20200115 |
| 1273292 | C01273292/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCIN; REGISTRATION NO/DATE: SWISSMEDIC 58751 28.05.2009 |
| 2929031 | C20210012 00398 | Estonia | ⤷ Try a Trial | PRODUCT NAME: INKLISIRAAN;REG NO/DATE: EU/1/20/1494 10.12.2020 |
| 2435024 | 21C1020 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI), GLYCOPYRROLATE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET BUDESONIDE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.