Bionpharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BIONPHARMA, and when can generic versions of BIONPHARMA drugs launch?
BIONPHARMA has sixty-nine approved drugs.
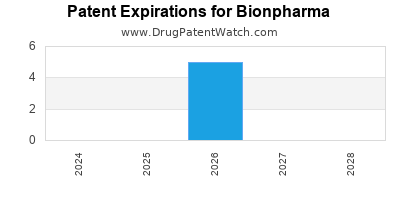
There are five US patents protecting BIONPHARMA drugs.
There are ten patent family members on BIONPHARMA drugs in thirteen countries and one hundred and ten supplementary protection certificates in fifteen countries.
Summary for Bionpharma
| International Patents: | 10 |
| US Patents: | 5 |
| Tradenames: | 64 |
| Ingredients: | 59 |
| NDAs: | 69 |
| Patent Litigation for Bionpharma: | See patent lawsuits for Bionpharma |
Drugs and US Patents for Bionpharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bionpharma | CETIRIZINE HYDROCHLORIDE ALLERGY | cetirizine hydrochloride | CAPSULE;ORAL | 022429-001 | Jul 23, 2009 | OTC | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bionpharma | MIDOL LIQUID GELS | ibuprofen | CAPSULE;ORAL | 021472-001 | Oct 18, 2002 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bionpharma | NICARDIPINE HYDROCHLORIDE | nicardipine hydrochloride | CAPSULE;ORAL | 217555-001 | May 3, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | SEVELAMER CARBONATE | sevelamer carbonate | FOR SUSPENSION;ORAL | 209374-001 | May 17, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | ZONISAMIDE | zonisamide | CAPSULE;ORAL | 077813-002 | Aug 16, 2006 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | DROXIDOPA | droxidopa | CAPSULE;ORAL | 213033-002 | Apr 28, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bionpharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bionpharma | MIDOL LIQUID GELS | ibuprofen | CAPSULE;ORAL | 021472-001 | Oct 18, 2002 | 6,251,426 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Bionpharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Slovenia | 1863458 | ⤷ Try a Trial |
| Cyprus | 1118321 | ⤷ Try a Trial |
| Poland | 1863458 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006096580 | ⤷ Try a Trial |
| Lithuania | 1863458 | ⤷ Try a Trial |
| European Patent Office | 1863458 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bionpharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0764174 | SPC/GB04/031 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: COLESEVELAM HYDROCHLORIDE; REGISTERED: UK EU/1/03/268/001-003 20040310 |
| 0716606 | C300080 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SEVELAMER, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AAN- VAARDBAAR ZOUT, IN HET BIJZONDER SEVELAMER HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/99/123/001-00420000128 20000128 |
| 0627406 | SPC/GB11/026 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FINGOLIMOD, I.E. 2-AMINO-2-(2-(4-OCTYLPHENYL)ETHYL)PROPANE-1,3-DIOL, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/11/677/001 20110317; UK EU/1/11/677/002 20110317; UK EU/1/11/677/003 20110317; UK EU/1/11/677/004 20110317 |
| 0719278 | SPC/GB03/018 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DUTASTERIDE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SOLVATE.; REGISTERED: SE 17871 20020719; SE 17872 20020719; UK PL 19494/0005 20030117; UK PL 19494/0006 20030117 |
| 1613288 | CR 2011 00023 | Denmark | ⤷ Try a Trial | PRODUCT NAME: FINGOLIMOD, HERUNDER FINGOLIMOD SOM HYDROCHLORID; REG. NO/DATE: EU/1/11/677/001-004 20110317 |
| 1110543 | 08C0004 | France | ⤷ Try a Trial | PRODUCT NAME: DESLORATADINE; PSEUDOEPHEDRINE SULFATE; REGISTRATION NO/DATE: EU/1/07/399/001 20070730 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.