XYREM Drug Patent Profile
✉ Email this page to a colleague
When do Xyrem patents expire, and when can generic versions of Xyrem launch?
Xyrem is a drug marketed by Jazz Pharms and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has thirty-three patent family members in twenty-one countries.
The generic ingredient in XYREM is sodium oxybate. There are one thousand four hundred and seventy-two drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the sodium oxybate profile page.
DrugPatentWatch® Generic Entry Outlook for Xyrem
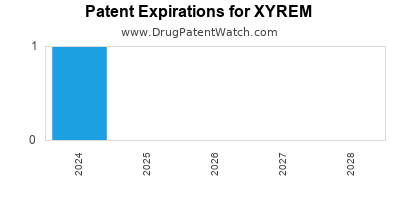
Xyrem was eligible for patent challenges on July 17, 2006.
There have been fifteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (sodium oxybate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for XYREM
| International Patents: | 33 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 29 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for XYREM |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for XYREM |
| What excipients (inactive ingredients) are in XYREM? | XYREM excipients list |
| DailyMed Link: | XYREM at DailyMed |

Pharmacology for XYREM
| Drug Class | Central Nervous System Depressant |
| Physiological Effect | Central Nervous System Depression Decreased Central Nervous System Organized Electrical Activity |
Anatomical Therapeutic Chemical (ATC) Classes for XYREM
Paragraph IV (Patent) Challenges for XYREM
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| XYREM | Oral Solution | sodium oxybate | 500 mg/mL | 021196 | 1 | 2010-07-08 |
US Patents and Regulatory Information for XYREM
XYREM is protected by seven US patents and two FDA Regulatory Exclusivities.
Patents protecting XYREM
Method of administration of gamma hydroxybutyrate with monocarboxylate transporters
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of administration of gamma hydroxybutyrate with monocarboxylate transporters
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of administration of gamma hydroxybutyrate with monocarboxylate transporters
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Sensitive drug distribution system and method
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of administration of gamma hydroxybutyrate with monocarboxylate transporters
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of administration of gamma hydroxybutyrate with monocarboxylate transporters
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of administration of gamma hydroxybutyrate with monocarboxylate transporters
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting XYREM
INDICATED FOR THE TREATMENT OF CATAPLEXY OR EXCESSIVE DAYTIME SLEEPINESS (EDS) IN PEDIATRIC PATIENTS 7 YEARS OF AGE AND OLDER WITH NARCOLEPSY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jazz Pharms | XYREM | sodium oxybate | SOLUTION;ORAL | 021196-001 | Jul 17, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Jazz Pharms | XYREM | sodium oxybate | SOLUTION;ORAL | 021196-001 | Jul 17, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Jazz Pharms | XYREM | sodium oxybate | SOLUTION;ORAL | 021196-001 | Jul 17, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Jazz Pharms | XYREM | sodium oxybate | SOLUTION;ORAL | 021196-001 | Jul 17, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Jazz Pharms | XYREM | sodium oxybate | SOLUTION;ORAL | 021196-001 | Jul 17, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for XYREM
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| UCB Pharma Ltd | Xyrem | sodium oxybate | EMEA/H/C/000593 Treatment of narcolepsy with cataplexy in adult patients. |
Authorised | no | no | no | 2005-10-13 | |
| D&A Pharma | Hopveus | sodium oxybate | EMEA/H/C/004962 Substitution treatment for alcohol dependence within a framework of careful medical supervision along with continuous psychosocial support and social rehabilitation. Treatment should be initiated only in patients resistant to existing interventions or in patients for whom existing therapies are contra-indicated or not recommended. |
Refused | no | no | no | 2020-07-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for XYREM
See the table below for patents covering XYREM around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 779354 | ⤷ Try a Trial | |
| Japan | 4374441 | ⤷ Try a Trial | |
| Israel | 240874 | מתן של גמא -הידרוקסיבוטיראט עם נשאי מונוקרבוקסילאט (Administration of gamma hydroxybutyrate with monocarboxylate transporters) | ⤷ Try a Trial |
| Croatia | P20200215 | ⤷ Try a Trial | |
| China | 111317730 | γ羟基丁酸与单羧酸转运蛋白的施用 (Administration of gamma hydroxybutyrate with monocarboxylate transporters) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for XYREM
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2563920 | CR 2019 00001 | Denmark | ⤷ Try a Trial | PRODUCT NAME: INOTERSEN AND SALT THEREOF, INCLUDING SODIUM SALTS; REG. NO/DATE: EU/1/18/1296 20180710 |
| 3141251 | 301099 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, SODIUM SULPHATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE AND THE SECOND DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, ASCORBIC ACID, SODIUM ASCORBATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE; NATIONAL REGISTRATION NO/DATE: RVG 120195 20171114; FIRST REGISTRATION: IS IS/1/17/083/01 20171016 |
| 0579826 | 02C0041 | France | ⤷ Try a Trial | PRODUCT NAME: ERTAPENEM SODIUM; REGISTRATION NO/DATE: EU/1/02/216/001 20020418 |
| 2203431 | 1590018-6 | Sweden | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150119 |
| 0268956 | SPC/GB98/040 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: RABEPRAZOLE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE SODIUM SALT; REGISTERED: UK 10555/0010 19980508; UK 10555/0008 19980508 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


