TRIAMTERENE Drug Patent Profile
✉ Email this page to a colleague
When do Triamterene patents expire, and what generic alternatives are available?
Triamterene is a drug marketed by Agnitio, Eywa, Ani Pharms, Cadila, Chartwell Rx, Duramed Pharms Barr, Lannett Co Inc, Mylan, Novartis, Sandoz, Vitarine, Am Therap, Apotex Inc, Pliva, Quantum Pharmics, Rubicon, Watson Labs, and Zydus Pharms. and is included in twenty-four NDAs.
The generic ingredient in TRIAMTERENE is hydrochlorothiazide; triamterene. There are thirty-two drug master file entries for this compound. Thirty suppliers are listed for this compound. Additional details are available on the hydrochlorothiazide; triamterene profile page.
Summary for TRIAMTERENE
| US Patents: | 0 |
| Applicants: | 18 |
| NDAs: | 24 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 130 |
| Clinical Trials: | 8 |
| Patent Applications: | 3,680 |
| Formulation / Manufacturing: | see details |
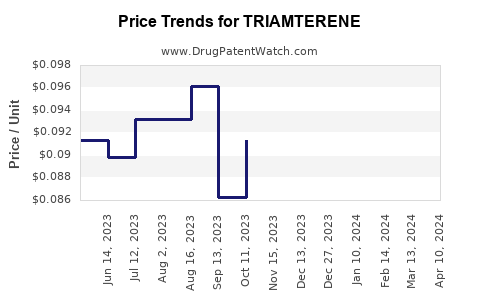
| Drug Prices: | Drug price information for TRIAMTERENE |
| Drug Sales Revenues: | Drug sales revenues for TRIAMTERENE |
| What excipients (inactive ingredients) are in TRIAMTERENE? | TRIAMTERENE excipients list |
| DailyMed Link: | TRIAMTERENE at DailyMed |
Recent Clinical Trials for TRIAMTERENE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of California, Irvine | Phase 4 |
| Vanderbilt University | Phase 4 |
| West China Hospital | Phase 4 |
Pharmacology for TRIAMTERENE
| Drug Class | Potassium-sparing Diuretic |
| Physiological Effect | Decreased Renal K+ Excretion Increased Diuresis |


