Icosapent ethyl - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for icosapent ethyl and what is the scope of freedom to operate?
Icosapent ethyl
is the generic ingredient in two branded drugs marketed by Apotex, Ascent Pharms Inc, Dr Reddys, Hikma, Humanwell Puracap, Strides Pharma, Teva Pharms Usa, Zydus, and Amarin Pharms, and is included in nine NDAs. There are seventy patents protecting this compound and three Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Icosapent ethyl has three hundred and forty-nine patent family members in forty-five countries.
There are six drug master file entries for icosapent ethyl. Fifteen suppliers are listed for this compound.
Summary for icosapent ethyl
| International Patents: | 349 |
| US Patents: | 70 |
| Tradenames: | 2 |
| Applicants: | 9 |
| NDAs: | 9 |
| Drug Master File Entries: | 6 |
| Finished Product Suppliers / Packagers: | 15 |
| Raw Ingredient (Bulk) Api Vendors: | 60 |
| Clinical Trials: | 17 |
| Patent Applications: | 2,462 |
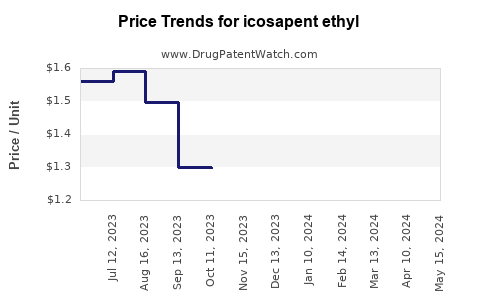
| Drug Prices: | Drug price trends for icosapent ethyl |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for icosapent ethyl |
| What excipients (inactive ingredients) are in icosapent ethyl? | icosapent ethyl excipients list |
| DailyMed Link: | icosapent ethyl at DailyMed |
Recent Clinical Trials for icosapent ethyl
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 1/Phase 2 |
| University of Western Ontario, Canada | Phase 4 |
| HLS Therapeutics, Inc | Phase 4 |
Paragraph IV (Patent) Challenges for ICOSAPENT ETHYL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VASCEPA | Capsules | icosapent ethyl | 500 mg | 202057 | 1 | 2017-08-29 |
| VASCEPA | Capsules | icosapent ethyl | 1 g | 202057 | 4 | 2016-07-26 |
US Patents and Regulatory Information for icosapent ethyl
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Teva Pharms Usa | ICOSAPENT ETHYL | icosapent ethyl | CAPSULE;ORAL | 209525-002 | Sep 11, 2020 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-002 | Feb 16, 2017 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-002 | Feb 16, 2017 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for icosapent ethyl
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-002 | Feb 16, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for icosapent ethyl
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Amarin Pharmaceuticals Ireland Limited | Vazkepa | icosapent ethyl | EMEA/H/C/005398 Indicated to reduce cardiovascular risk as an adjunct to statin therapy. |
Authorised | no | no | no | 2021-03-26 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for icosapent ethyl
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20110110321 | USE OF EICOSAPENTAENOIC ACID ETHYL ESTER FOR TREATING HYPERTRIGLYCERIDEMIA | ⤷ Try a Trial |
| Brazil | PI1014405 | composições farmacêuticas compreendendo epa e um agente cardiovascular e métodos de seu uso | ⤷ Try a Trial |
| European Patent Office | 3791880 | COMPOSITIONS PHARMACEUTIQUES COMPRENANT DE L'EPA (PHARMACEUTICAL COMPOSITIONS COMPRISING EPA) | ⤷ Try a Trial |
| China | 106074486 | 在相伴他汀类疗法的对象中降低甘油三酯、没有增加LDL‑C水平的组合物和方法 (Compositions And Methods For Lowering Triglycerides Without Raising Ldl-C Levels In A Subject On Concomitant Statin Therapy) | ⤷ Try a Trial |
| Russian Federation | 2020101129 | СТАБИЛЬНЫЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ И СПОСОБЫ ИХ ПРИМЕНЕНИЯ | ⤷ Try a Trial |
| Japan | 2007039452 | COMPOSITION FOR PREVENTING OCCURRENCE OF CARDIOVASCULAR EVENT | ⤷ Try a Trial |
| Poland | 2395991 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for icosapent ethyl
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2443246 | C202130052 | Spain | ⤷ Try a Trial | PRODUCT NAME: ICOSAPENTO DE ETILO; NATIONAL AUTHORISATION NUMBER: EU/1/20/1524; DATE OF AUTHORISATION: 20210326; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1524; DATE OF FIRST AUTHORISATION IN EEA: 20210326 |
| 2022495 | 21C1045 | France | ⤷ Try a Trial | PRODUCT NAME: ICOSAPENT ETHYL; REGISTRATION NO/DATE: EU/1/20/1524 20210329 |
| 2443246 | 122021000056 | Germany | ⤷ Try a Trial | PRODUCT NAME: VAZKEPA (ICOSAPENT ETHYL); REGISTRATION NO/DATE: EU/1/20/1524 20210326 |
| 2443246 | PA2021522 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IKOZAPENTO ETILAS; REGISTRATION NO/DATE: EU/1/20/1524 20210326 |
| 2443246 | C20210022 00395 | Estonia | ⤷ Try a Trial | PRODUCT NAME: IKOSAPENTETUEUEL;REG NO/DATE: EU/1/20/1524 29.03.2021 |
| 2022495 | 132021000000154 | Italy | ⤷ Try a Trial | PRODUCT NAME: ICOSAPENT ETILE(VAZKEPA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1524, 20210329 |
| 2443246 | 21C1046 | France | ⤷ Try a Trial | PRODUCT NAME: ICOSAPENT ETHYL; NAT. REGISTRATION NO/DATE: EU/1/20/1524 20210329; FIRST REGISTRATION: - EU/1/20/1524 20210329 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |