Zr Pharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ZR PHARMA, and when can generic versions of ZR PHARMA drugs launch?
ZR PHARMA has one approved drug.
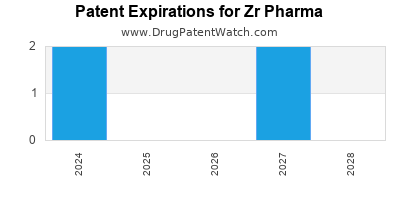
There are eleven US patents protecting ZR PHARMA drugs.
There are two hundred and six patent family members on ZR PHARMA drugs in forty-two countries and thirty-one supplementary protection certificates in eighteen countries.
Drugs and US Patents for Zr Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-003 | May 1, 2017 | RX | Yes | No | 7,351,701 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-002 | Dec 19, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-002 | Dec 19, 2016 | RX | Yes | Yes | 7,531,530 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-002 | Dec 19, 2016 | RX | Yes | Yes | 9,987,285 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-002 | Dec 19, 2016 | RX | Yes | Yes | 8,754,072 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Zr Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-001 | Dec 19, 2016 | 6,495,541 | ⤷ Try a Trial |
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-003 | May 1, 2017 | 6,495,541 | ⤷ Try a Trial |
| Zr Pharma | RUBRACA | rucaparib camsylate | TABLET;ORAL | 209115-002 | Dec 19, 2016 | 6,495,541 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Zr Pharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Singapore | 10201406805R | ⤷ Try a Trial |
| Singapore | 11201700265V | ⤷ Try a Trial |
| Canada | 2533332 | ⤷ Try a Trial |
| Germany | 602004032537 | ⤷ Try a Trial |
| Denmark | 1684736 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Zr Pharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1633724 | 596 | Finland | ⤷ Try a Trial | |
| 1633724 | 2015C/024 | Belgium | ⤷ Try a Trial | PRODUCT NAME: OLAPARIB ET LES SELS ET SOLVATES DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/14/959 20141218 |
| 1633724 | PA2015016,C1633724 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: OLAPARIBAS IR JO DRUSKOS IR SOLVATAI; REGISTRATION NO/DATE: EU/1/14/959 20141216 |
| 2534153 | 1890042-3 | Sweden | ⤷ Try a Trial | PRODUCT NAME: RUCAPARIB CAMSYLATE; REG. NO/DATE: EU/1/17/1250 20180529 |
| 1633724 | C 2015 011 | Romania | ⤷ Try a Trial | PRODUCT NAME: OLAPARIB; NATIONAL AUTHORISATION NUMBER: EU/1/14/959/001; DATE OF NATIONAL AUTHORISATION: 20141216; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/14/959/001; DATE OF FIRST AUTHORISATION IN EEA: 20141216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.