Xspire Pharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for XSPIRE PHARMA, and what generic alternatives to XSPIRE PHARMA drugs are available?
XSPIRE PHARMA has six approved drugs.
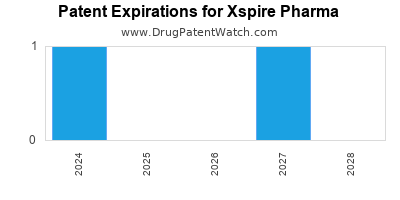
There are two US patents protecting XSPIRE PHARMA drugs.
There are one hundred and fifty-five patent family members on XSPIRE PHARMA drugs in thirty-seven countries and twenty-two supplementary protection certificates in sixteen countries.
Drugs and US Patents for Xspire Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xspire Pharma | NALFON | fenoprofen calcium | CAPSULE;ORAL | 017604-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Xspire Pharma | FENOPROFEN CALCIUM | fenoprofen calcium | TABLET;ORAL | 072267-001 | Aug 17, 1988 | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Xspire Pharma | NALFON | fenoprofen calcium | CAPSULE;ORAL | 017604-003 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Xspire Pharma | ZONTIVITY | vorapaxar sulfate | TABLET;ORAL | 204886-001 | May 8, 2014 | RX | Yes | Yes | 7,713,999 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Xspire Pharma | NALFON | fenoprofen calcium | CAPSULE;ORAL | 017604-004 | Jul 21, 2009 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Xspire Pharma | BUTALBITAL, ACETAMINOPHEN AND CAFFEINE | acetaminophen; butalbital; caffeine | CAPSULE;ORAL | 206615-001 | Aug 4, 2017 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Xspire Pharma | TREZIX | acetaminophen; caffeine; dihydrocodeine bitartrate | CAPSULE;ORAL | 204785-001 | Nov 26, 2014 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Xspire Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Xspire Pharma | ZONTIVITY | vorapaxar sulfate | TABLET;ORAL | 204886-001 | May 8, 2014 | 7,235,567 | ⤷ Try a Trial |
| Xspire Pharma | NALFON | fenoprofen calcium | CAPSULE;ORAL | 017604-003 | Approved Prior to Jan 1, 1982 | 3,600,437 | ⤷ Try a Trial |
| Xspire Pharma | NALFON | fenoprofen calcium | CAPSULE;ORAL | 017604-002 | Approved Prior to Jan 1, 1982 | 3,600,437 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Xspire Pharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2005528406 | ⤷ Try a Trial |
| Russian Federation | 2004133375 | ⤷ Try a Trial |
| Colombia | 5680428 | ⤷ Try a Trial |
| Japan | 2007523051 | ⤷ Try a Trial |
| Norway | 20025965 | ⤷ Try a Trial |
| China | 1878552 | ⤷ Try a Trial |
| Argentina | 045809 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Xspire Pharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1429780 | 13C0012 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE CIPROFLOXACINE ET DE DEXAMETHASONE, EN PARTICULIER DE CHLORHYDRATE DE CIPROFLOXACINE ET DE DEXAMETHASONE; NAT. REGISTRATION NO/DATE: NL 41308 20121214; FIRST REGISTRATION: 48976 20120808 |
| 1495018 | 218 50011-2015 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: VORAPAXARIUMSULFAT; REGISTRATION NO/DATE: EU/1/14/976/001 - EU/1/14/976/006 20150121 |
| 1495018 | CA 2015 00037 | Denmark | ⤷ Try a Trial | PRODUCT NAME: VORAPAXAR, OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF, INCLUDING VORAPAXARSULPHATE; REG. NO/DATE: EU/1/14/976/001-06 20150119 |
| 1495018 | 1590037-6 | Sweden | ⤷ Try a Trial | PRODUCT NAME: VORAPAXAR, OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF; REG. NO/DATE: EU/1/14/976/001 20150121 |
| 1495018 | 15C0047 | France | ⤷ Try a Trial | PRODUCT NAME: VORAPAXAR,OU L'UN DE SES SELS OU SOLVATES PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/14/976/001-006 20150121 |
| 1495018 | 2015C/037 | Belgium | ⤷ Try a Trial | PRODUCT NAME: VORAPAXAR, OU SON SEL OU SOLVAT PHARMACOLOGIQUEMENT ADMISSIBLE; AUTHORISATION NUMBER AND DATE: EU/1/14/976/001 |
| 1495018 | 2015/035 | Ireland | ⤷ Try a Trial | PRODUCT NAME: VORAPAXAR, OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF; REGISTRATION NO/DATE: EU/1/14/976/001-006 20150119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.