Wyeth Pharms Company Profile
✉ Email this page to a colleague
What is the competitive landscape for WYETH PHARMS, and what generic alternatives to WYETH PHARMS drugs are available?
WYETH PHARMS has thirty-one approved drugs.
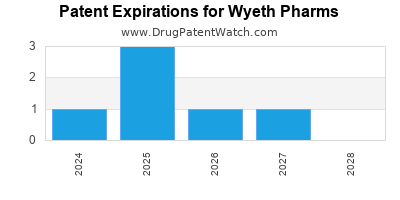
There are six US patents protecting WYETH PHARMS drugs.
There are fifty-six patent family members on WYETH PHARMS drugs in thirty countries and thirty-six supplementary protection certificates in thirteen countries.
Drugs and US Patents for Wyeth Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wyeth Pharms | OVRAL | ethinyl estradiol; norgestrel | TABLET;ORAL-21 | 016672-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms | PREMARIN | estrogens, conjugated | TABLET;ORAL | 004782-003 | Approved Prior to Jan 1, 1982 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms Inc | CORDARONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 020377-001 | Aug 3, 1995 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Wyeth Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Wyeth Pharms | PROTONIX | pantoprazole sodium | TABLET, DELAYED RELEASE;ORAL | 020987-001 | Feb 2, 2000 | 4,758,579*PED | ⤷ Try a Trial |
| Wyeth Pharms | PREMPHASE 14/14 | estrogens, conjugated; medroxyprogesterone acetate | TABLET;ORAL-28 | 020527-002 | Nov 17, 1995 | RE36247 | ⤷ Try a Trial |
| Wyeth Pharms Inc | EFFEXOR | venlafaxine hydrochloride | TABLET;ORAL | 020151-004 | Dec 28, 1993 | 5,916,923 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for WYETH PHARMS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 20 mg and 40 mg | ➤ Subscribe | 2004-02-02 |
| ➤ Subscribe | Tablets | 25 mg, 37.5 mg, 50 mg, 75 mg and 100 mg | ➤ Subscribe | 2005-11-03 |
| ➤ Subscribe | For Injection | 12 g/1.5 g per vial (pharmacy bulk) | ➤ Subscribe | 2011-12-06 |
| ➤ Subscribe | Tablets | 0.09 mg/0.02 mg | ➤ Subscribe | 2007-10-05 |
International Patents for Wyeth Pharms Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Serbia | 54699 | ⤷ Try a Trial |
| Malaysia | 147180 | ⤷ Try a Trial |
| European Patent Office | 1667660 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Wyeth Pharms Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | C201630040 | Spain | ⤷ Try a Trial | PRODUCT NAME: ETINILESTRADIOL Y MEZCLA DE LEVONORGESTREL Y ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: 80340; DATE OF AUTHORISATION: 20160122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150211 |
| 1453521 | 93156 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL ET ETHINYLESTRADIOL; FIRST REGISTRATION DATE: 20150211 |
| 0802183 | SPC/GB09/045 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: BAZEDOXIFENE AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU/1/09/511/001 20090417; UK EU/1/09/511/002 20090417; UK EU/1/09/511/003 20090417; UK EU/1/09/511/004 20090417 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.