Twi Pharms Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TWI PHARMS, and when can generic versions of TWI PHARMS drugs launch?
TWI PHARMS has thirty-two approved drugs.
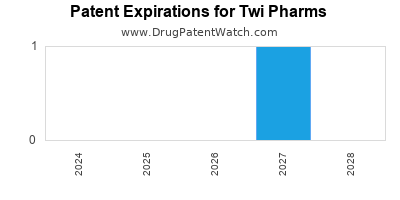
There is one US patent protecting TWI PHARMS drugs. There is one tentative approval on TWI PHARMS drugs.
There is one patent family member on TWI PHARMS drugs in one country and one hundred and thirty-three supplementary protection certificates in sixteen countries.
Drugs and US Patents for Twi Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Twi Pharms | ATENOLOL | atenolol | TABLET;ORAL | 072304-002 | Jul 31, 1992 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Twi Pharms | ZESTRIL | lisinopril | TABLET;ORAL | 019777-005 | Apr 29, 1993 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Twi Pharms | DILTIAZEM HYDROCHLORIDE | diltiazem hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 205231-003 | Aug 30, 2018 | AB3 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Twi Pharms | TESTOSTERONE | testosterone | SOLUTION, METERED;TRANSDERMAL | 209836-001 | Sep 3, 2021 | AT | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Twi Pharms | DICLOFENAC SODIUM | diclofenac sodium | SOLUTION;TOPICAL | 202393-001 | Nov 24, 2014 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Twi Pharms | ZESTRIL | lisinopril | TABLET;ORAL | 019777-001 | May 19, 1988 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Twi Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Twi Pharms | TENORETIC 50 | atenolol; chlorthalidone | TABLET;ORAL | 018760-002 | Jun 8, 1984 | 3,663,607 | ⤷ Try a Trial |
| Twi Pharms | NAPRELAN | naproxen sodium | TABLET, EXTENDED RELEASE;ORAL | 020353-003 | Jan 5, 1996 | 5,637,320 | ⤷ Try a Trial |
| Twi Pharms | ZESTRIL | lisinopril | TABLET;ORAL | 019777-003 | May 19, 1988 | 4,374,829*PED | ⤷ Try a Trial |
| Twi Pharms | NAPRELAN | naproxen sodium | TABLET, EXTENDED RELEASE;ORAL | 020353-001 | Jan 5, 1996 | 5,637,320 | ⤷ Try a Trial |
| Twi Pharms | TENORMIN | atenolol | TABLET;ORAL | 018240-004 | Apr 9, 1990 | 3,836,671 | ⤷ Try a Trial |
| Twi Pharms | TENORMIN | atenolol | TABLET;ORAL | 018240-001 | Approved Prior to Jan 1, 1982 | 3,663,607 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TWI PHARMS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 450 mg | ➤ Subscribe | 2013-02-28 |
International Patents for Twi Pharms Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2008038155 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Twi Pharms Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0605697 | SPC/GB01/008 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: 1-(((3-(2-(DIMETHYLAMINO)ETHYL)INDOL-5-YL)METHYL)SULFONYL)PYRROLIDINE GENERIC NAME ALMOTRIPTAN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, ESPECIALLY AS ALMOTRIPTAN D,L - HYDROGEN MALATE; REGISTERED: ES 62.877 19991223; UK PL 16973/0005 20001026 |
| 2137537 | 92487 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: DIMETHYL FUMARATE. FIRST REGISTRATION: 20140203 |
| 1412357 | PA2008013,C1412357 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINUM PHOSPHAS MONOHYDRICUS, METFORMINI HYDROCHLORIDUM; REGISTRATION NO/DATE: EU/1/08/455/001 - EU/1/08/455/014 20080716 |
| 2316456 | 17C1058 | France | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER CHLORHYDRATE DE NALTREXONE ET,BUPROPION OU SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER CHLORHYDRATE DE BUPROPION; REGISTRATION NO/DATE: EU/1/14/988 20150330 |
| 1532149 | CA 2013 00001 | Denmark | ⤷ Try a Trial | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2-YLMETHYL)-3,7-DIHYDROPURIN-2,6-DION ENANTIOMERER OG SALTE DERAF - SAERLIGT LINAGLIPTIN - I KOMBINATION MED METFORMINHYDROCHLORID; REG. NO/DATE: EU/1/12/780/001-028 20120720 |
| 2653873 | 2023C/501 | Belgium | ⤷ Try a Trial | PRODUCT NAME: FUMARATE DE DIMETHYLE; AUTHORISATION NUMBER AND DATE: EU/1/13/837 20140203 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.