Teva Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TEVA, and when can generic versions of TEVA drugs launch?
TEVA has seven hundred and thirty-six approved drugs.
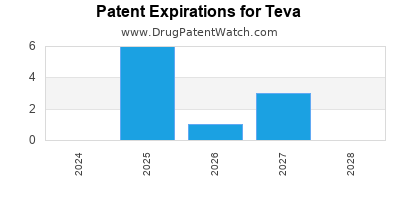
There are one hundred US patents protecting TEVA drugs. There are fifty-three tentative approvals on TEVA drugs.
There are nine hundred and thirteen patent family members on TEVA drugs in forty-nine countries and one thousand and fifty-six supplementary protection certificates in eighteen countries.
Summary for Teva
| International Patents: | 913 |
| US Patents: | 100 |
| Tradenames: | 508 |
| Ingredients: | 443 |
| NDAs: | 736 |
| Patent Litigation for Teva: | See patent lawsuits for Teva |
| PTAB Cases with Teva as petitioner: | See PTAB cases with Teva as petitioner |
Drugs and US Patents for Teva
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Pharms Usa | PARICALCITOL | paricalcitol | CAPSULE;ORAL | 090829-002 | Sep 27, 2013 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva Pharms | DEXMETHYLPHENIDATE HYDROCHLORIDE | dexmethylphenidate hydrochloride | TABLET;ORAL | 077107-003 | Jan 29, 2007 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Branded Pharm | AUSTEDO | deutetrabenazine | TABLET;ORAL | 208082-001 | Apr 3, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms Usa | DOFETILIDE | dofetilide | CAPSULE;ORAL | 210018-002 | Apr 15, 2022 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Teva
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Pharms Intl | AMRIX | cyclobenzaprine hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021777-002 | Feb 1, 2007 | 9,375,410 | ⤷ Try a Trial |
| Teva Branded Pharm | LOESTRIN 24 FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL | 021871-001 | Feb 17, 2006 | 5,552,394 | ⤷ Try a Trial |
| Teva Womens | PREFEST | estradiol; norgestimate | TABLET;ORAL | 021040-001 | Oct 22, 1999 | 7,320,970 | ⤷ Try a Trial |
| Teva Branded Pharm | LOXITANE C | loxapine hydrochloride | CONCENTRATE;ORAL | 017658-001 | Approved Prior to Jan 1, 1982 | 3,546,226 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TEVA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.5 mg and 1 mg | ➤ Subscribe | 2010-05-17 |
| ➤ Subscribe | Extended-release Capsule | 15 mg and 30 mg | ➤ Subscribe | 2008-08-11 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-01-29 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg | ➤ Subscribe | 2004-03-29 |
| ➤ Subscribe | Tablets | 0.15 mg/0.02 mg, 0.15 mg/0.025 mg, 0.15 mg/0.03 mg and 0.01 mg | ➤ Subscribe | 2013-07-10 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2006-04-17 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 20 mg, 30 mg | ➤ Subscribe | 2009-11-18 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-02-26 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg/0.01 mg | ➤ Subscribe | 2008-01-22 |
| ➤ Subscribe | Tablets | 0.1 mg/0.02 mg and 0.01 mg | ➤ Subscribe | 2009-11-16 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | for Injection | 3.5 mg/vial | ➤ Subscribe | 2016-10-26 |
Premature patent expirations for TEVA
Expiration due to failure to pay maintenance fee
| Patent Number | Expiration Date |
|---|---|
| ⤷ Try a Trial | ⤷ Try a Trial |
International Patents for Teva Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2005070332 | ⤷ Try a Trial |
| Singapore | 191414 | ⤷ Try a Trial |
| Eurasian Patent Organization | 023339 | ⤷ Try a Trial |
| Canada | 2575957 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Teva Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435025 | 122019000068 | Germany | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON GLYCOPYRROLAT, EINSCHLIESSLICH BELIEBIGER PHARMAZEUTISCH VERTRAEGLICHER SALZE, ESTER, ENANTIOMERE, ODER ANDERER DERIVATE DAVON, UND FORMOTEROL, EINSCHLIESSLICH BELIEBIGER PHARMAZEUTISCH VERTRAEGLICHER SALZE, ESTER, ENANTIOMERE, ODER ANDERER DERIVATE DAVON; REGISTRATION NO/DATE: EU/1/18/1339 20181218 |
| 1746976 | 17C1027 | France | ⤷ Try a Trial | PRODUCT NAME: SEL DE SUCROSOFATE D'IRINOTECAN; REGISTRATION NO/DATE: EU/1/16/1130 20161018 |
| 1506211 | 300677 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN METFORMINE OF EEN FARMACEITISCH AANVAARDBAAR ZOUT DAARVAN, ZOALS BESCHERMD DOOR HET BASISOCTROOI EP 1506211B1; REGISTRATION NO/DATE: EU/1/13/900 20140121 |
| 2203462 | 132014902310732 | Italy | ⤷ Try a Trial | PRODUCT NAME: SOFOSBUVIR(SOVALDI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/894, 20140117 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.