Pharmacyclics Llc Company Profile
✉ Email this page to a colleague
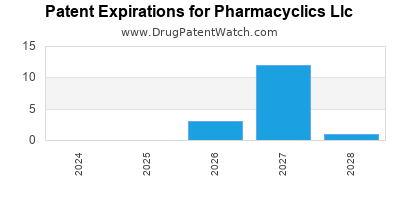
What is the competitive landscape for PHARMACYCLICS LLC, and when can generic versions of PHARMACYCLICS LLC drugs launch?
PHARMACYCLICS LLC has three approved drugs.
There are forty-one US patents protecting PHARMACYCLICS LLC drugs.
There are three hundred and forty-seven patent family members on PHARMACYCLICS LLC drugs in forty-six countries and thirty-four supplementary protection certificates in fifteen countries.
Summary for Pharmacyclics Llc
| International Patents: | 347 |
| US Patents: | 41 |
| Tradenames: | 1 |
| Ingredients: | 1 |
| NDAs: | 3 |
| Patent Litigation for Pharmacyclics Llc: | See patent lawsuits for Pharmacyclics Llc |
Drugs and US Patents for Pharmacyclics Llc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | TABLET;ORAL | 210563-002 | Feb 16, 2018 | RX | Yes | No | 10,653,696*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | TABLET;ORAL | 210563-001 | Feb 16, 2018 | RX | Yes | No | 9,795,604*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | TABLET;ORAL | 210563-004 | Feb 16, 2018 | DISCN | Yes | No | 10,961,251*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | CAPSULE;ORAL | 205552-002 | Dec 20, 2017 | RX | Yes | No | 10,478,439*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | CAPSULE;ORAL | 205552-002 | Dec 20, 2017 | RX | Yes | No | 8,703,780*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | SUSPENSION;ORAL | 217003-001 | Aug 24, 2022 | RX | Yes | Yes | 8,957,079*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pharmacyclics Llc | IMBRUVICA | ibrutinib | SUSPENSION;ORAL | 217003-001 | Aug 24, 2022 | RX | Yes | Yes | 7,514,444*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for PHARMACYCLICS LLC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 140 mg and 560 mg | ➤ Subscribe | 2018-11-05 |
| ➤ Subscribe | Capsules | 70 mg | ➤ Subscribe | 2018-12-14 |
| ➤ Subscribe | Tablets | 280 mg and 420 mg | ➤ Subscribe | 2018-12-14 |
International Patents for Pharmacyclics Llc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 106102745 | ⤷ Try a Trial |
| New Zealand | 579911 | ⤷ Try a Trial |
| South Africa | 201007561 | ⤷ Try a Trial |
| Singapore | 10202101389T | ⤷ Try a Trial |
| Israel | 300955 | ⤷ Try a Trial |
| Eurasian Patent Organization | 201300246 | ⤷ Try a Trial |
| Brazil | 112012030625 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Pharmacyclics Llc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2201840 | SPC/GB15/022 | United Kingdom | ⤷ Try a Trial | CORRECTION OF GRANT INFORMATION ON SUPPLEMENTARY PROTECTION CERTIFICATE APPLICATIONS APPLICANT: PHARMACYCLICS LLC995 EAST ARQUES AVENUE, SUNNYVALE, CA 94085, UNITED STATES OF AMERICA PRODUCT: IBRUTINIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF PRODUCT TYPE: MEDICINALAUTHORISED: UK EU/1/14/945 23 OCTOBER 2014 AUTHORISED EXTENSION: PATENT NO: EP2201840TITLE: INHIBITORS OF BRUTON'S TYROSINE KINASESPC NO: SPC/GB15/022DATE GRANTED: 15 OCTOBER 2020 MAXIMUM PERIOD EXPIRES ON: 22 OCTOBER 2029*CORRECTION OF GRANT DETAILS IN JOURNAL NUMBER 6860 DATED 11 NOVEMBER 2020 TO INCLUDE MAXIMUM EXPIRY DETAILS. |
| 2201840 | 2015/020 | Ireland | ⤷ Try a Trial | PRODUCT NAME: IBRUTINIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTRATION NO/DATE: EU/1/14/945 20141021 |
| 2201840 | PA2015017 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IBRUTINIBUM; REGISTRATION NO/DATE: EU/1/14/945 20141021 |
| 2529621 | LUC00011 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: IBRUTINIB, OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/14/945 20150707 |
| 2201840 | 122015000027 | Germany | ⤷ Try a Trial | PRODUCT NAME: LBRUTINIB ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/14/945 20141021 |
| 2526934 | C20160038 00313 | Estonia | ⤷ Try a Trial | PRODUCT NAME: IBRUTINIIB;REG NO/DATE: EU/1/14/945 30.05.2016 |
| 2529621 | PA2017009 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IBRUTINIBAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/14/945 C(2015)4704 20170703 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.