Otsuka Company Profile
✉ Email this page to a colleague
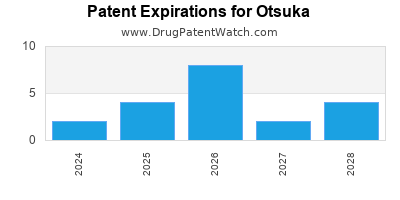
What is the competitive landscape for OTSUKA, and when can generic versions of OTSUKA drugs launch?
OTSUKA has seventeen approved drugs.
There are fifty-six US patents protecting OTSUKA drugs.
There are one thousand and seventeen patent family members on OTSUKA drugs in fifty-four countries and forty-nine supplementary protection certificates in sixteen countries.
Summary for Otsuka
| International Patents: | 1017 |
| US Patents: | 56 |
| Tradenames: | 15 |
| Ingredients: | 10 |
| NDAs: | 17 |
| Patent Litigation for Otsuka: | See patent lawsuits for Otsuka |
Drugs and US Patents for Otsuka
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-005 | Jul 10, 2015 | AB | RX | Yes | No | RE48059 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-006 | Nov 13, 2017 | RX | Yes | No | 10,441,194 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-002 | Jul 10, 2015 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Otsuka | SAMSCA | tolvaptan | TABLET;ORAL | 022275-001 | May 19, 2009 | AB | RX | Yes | No | 10,905,694 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Otsuka | ABILIFY ASIMTUFII | aripiprazole | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 217006-001 | Apr 27, 2023 | RX | Yes | No | 8,338,427 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka | JYNARQUE | tolvaptan | TABLET;ORAL | 204441-004 | Apr 23, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Otsuka
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-004 | Sep 29, 2014 | 5,006,528*PED | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET, ORALLY DISINTEGRATING;ORAL | 021729-004 | Jun 7, 2006 | 8,642,760*PED | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET, ORALLY DISINTEGRATING;ORAL | 021729-002 | Jun 7, 2006 | 9,089,567 | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET;ORAL | 021436-003 | Nov 15, 2002 | 9,387,182 | ⤷ Try a Trial |
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-002 | Nov 13, 2017 | 8,642,760 | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET;ORAL | 021436-003 | Nov 15, 2002 | 8,580,796*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for OTSUKA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 2 mg, 5 mg, 10 mg, 15 mg, 20 mg and 30 mg | ➤ Subscribe | 2006-11-15 |
| ➤ Subscribe | Tablets | 15 mg and 30 mg | ➤ Subscribe | 2013-09-23 |
| ➤ Subscribe | Injection | 6 mg/mL | ➤ Subscribe | 2012-12-26 |
| ➤ Subscribe | Oral Solution | 1 mg/mL | ➤ Subscribe | 2007-12-20 |
| ➤ Subscribe | Orally Disintegrating Tablets | 10 mg, 15 mg, 20 mg and 30 mg | ➤ Subscribe | 2006-11-15 |
| ➤ Subscribe | Tablets | 60 mg | ➤ Subscribe | 2018-03-26 |
International Patents for Otsuka Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 106692151 | ⤷ Try a Trial |
| Japan | 2017188914 | ⤷ Try a Trial |
| Poland | 2667856 | ⤷ Try a Trial |
| Luxembourg | C00086 | ⤷ Try a Trial |
| Poland | 393603 | ⤷ Try a Trial |
| Canada | 2780361 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Otsuka Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1675573 | C01675573/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: ARIPIPRAZOL; REGISTRATION NO/DATE: SWISSMEDIC 63177 28.04.2014 |
| 1869025 | 18C0004 | France | ⤷ Try a Trial | PRODUCT NAME: BREXPIPRAZOLE OU L'UN DE SES SELS; REGISTRATION NO/DATE: EU/1/18/1294 20180730 |
| 2207786 | LUC00327 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: CEDAZURIDINE OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; AUTHORISATION NUMBER AND DATE: EU/1/23/1756 20230918 |
| 1869025 | LUC00086 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: BREXPIPRAZOLE OU UN SEL DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/18/1294 20180730 |
| 0450097 | SPC/GB09/037 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TOLVAPTAN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: UK EU/1/09/539/001 20090803; UK EU/1/09/539/002 20090803; UK EU/1/09/539/003 20090803; UK EU/1/09/539/004 20090803 |
| 1869025 | 122018000088 | Germany | ⤷ Try a Trial | PRODUCT NAME: BREXPIPRAZOL ODER EIN SALZ DAVON; REGISTRATION NO/DATE: EU/1/18/1294 20180726 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.