Organon Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ORGANON, and what generic alternatives to ORGANON drugs are available?
ORGANON has fifty-nine approved drugs.
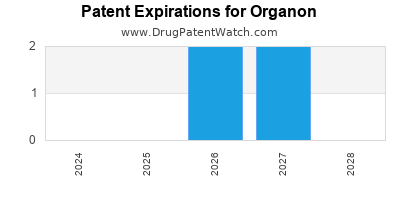
There are six US patents protecting ORGANON drugs. There is one tentative approval on ORGANON drugs.
There are three hundred and thirteen patent family members on ORGANON drugs in forty-four countries and one hundred and thirty-six supplementary protection certificates in fifteen countries.
Drugs and US Patents for Organon
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organon | ZOCOR | simvastatin | TABLET;ORAL | 019766-003 | Dec 23, 1991 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon | ELOCON | mometasone furoate | CREAM;TOPICAL | 019625-002 | Apr 19, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Organon Llc | XACIATO | clindamycin phosphate | GEL;VAGINAL | 215650-001 | Dec 7, 2021 | RX | Yes | Yes | 11,129,896 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon Usa Inc | NORCURON | vecuronium bromide | INJECTABLE;INJECTION | 018776-002 | Apr 30, 1984 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Organon | CELESTONE SOLUSPAN | betamethasone acetate; betamethasone sodium phosphate | INJECTABLE;INJECTION | 014602-001 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon Llc | ASMANEX TWISTHALER | mometasone furoate | POWDER;INHALATION | 021067-002 | Feb 1, 2008 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Organon Usa Inc | NORCURON | vecuronium bromide | INJECTABLE;INJECTION | 018776-003 | Jan 3, 1992 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Organon
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon | DIPROLENE | betamethasone dipropionate | LOTION, AUGMENTED;TOPICAL | 019716-001 | Aug 1, 1988 | 4,775,529 | ⤷ Try a Trial |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-002 | Jul 23, 2004 | RE37721*PED | ⤷ Try a Trial |
| Organon | LOTRISONE | betamethasone dipropionate; clotrimazole | CREAM;TOPICAL | 018827-001 | Jul 10, 1984 | 3,705,172 | ⤷ Try a Trial |
| Organon | COZAAR | losartan potassium | TABLET;ORAL | 020386-003 | Oct 13, 1998 | 5,608,075*PED | ⤷ Try a Trial |
| Organon Llc | ASMANEX HFA | mometasone furoate | AEROSOL, METERED;INHALATION | 205641-002 | Apr 25, 2014 | 6,057,307*PED | ⤷ Try a Trial |
| Organon | PROPECIA | finasteride | TABLET;ORAL | 020788-001 | Dec 19, 1997 | 5,571,817 | ⤷ Try a Trial |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-002 | Jul 23, 2004 | RE42461*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ORGANON drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 50 mg/12.5 mg and 100 mg/25 mg | ➤ Subscribe | 2004-05-24 |
| ➤ Subscribe | Tablets | 10 mg/10 mg, 10 mg/20 mg, 10 mg/40 mg and 10 mg/80 mg | ➤ Subscribe | 2009-07-27 |
| ➤ Subscribe | Nasal Spray | 50 mcg/ Spray | ➤ Subscribe | 2009-08-07 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2004-09-02 |
| ➤ Subscribe | Vaginal Ring | 0.015 mg/24 hour and 0.12 mg/24 hour | ➤ Subscribe | 2013-06-17 |
| ➤ Subscribe | Topical Solution (Lotion) | 0.10% | ➤ Subscribe | 2004-06-10 |
| ➤ Subscribe | Chewable Tablets | 4 mg and 5 mg | ➤ Subscribe | 2006-12-26 |
| ➤ Subscribe | Orally Disintegrating Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2006-06-21 |
| ➤ Subscribe | Oral Solution | 70 mg/75 mL | ➤ Subscribe | 2007-09-07 |
| ➤ Subscribe | Tablets | 100 mg/12.5 mL | ➤ Subscribe | 2006-04-04 |
| ➤ Subscribe | Tablets | 10 mg | ➤ Subscribe | 2006-10-25 |
| ➤ Subscribe | Tablets | 70 mg/2800 IU and 70 mg/5600 IU | ➤ Subscribe | 2007-11-20 |
| ➤ Subscribe | Injection | 250 mcg/0.5 mL, 1 mL PFS | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | Tablets | 10 mg | ➤ Subscribe | 2007-02-20 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2006-06-21 |
| ➤ Subscribe | Orally Disintegrating Tablets | 5 mg and 10 mg | ➤ Subscribe | 2006-02-17 |
International Patents for Organon Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2009286801 | ⤷ Try a Trial |
| Australia | 2002247019 | ⤷ Try a Trial |
| Spain | 2308460 | ⤷ Try a Trial |
| Slovenia | 1413331 | ⤷ Try a Trial |
| Hungary | E052622 | ⤷ Try a Trial |
| Brazil | PI0706472 | ⤷ Try a Trial |
| Brazil | 0206654 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Organon Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0454511 | 99C0009 | Belgium | ⤷ Try a Trial | PRODUCT NAME: IRBESARTAN / HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/98/086/001 19981015 |
| 0613371 | 2002C/022 | Belgium | ⤷ Try a Trial | PRODUCT NAME: BUDESONID. MICRONIS. AND FORMOTEROL. FUMARAS DIHYDR., NATL REGISTRATION NO/DATE: 624 IS 234 F 0 20010129; FIRST REGISTRATION: SE 16047 20000825 |
| 0480717 | 9890027-7 | Sweden | ⤷ Try a Trial | PRODUCT NAME: MONTELUKAST, R-(E)-1-1-3-2-(7-KLOR-2KINOLINYL)-ETENYL)-FENYL)-3-(2-(1-HYDROXI-1-METYLETYL)-FENYL)-PROPYL)-TIO)METYL)-CYKLOPROPANAETTIKSYRA, ELLER ETT FARMACEUTISKT GODDTAGBART SALT DAERAV, FOERETRAEDESVIS NATRIUMSALTET; NAT REG./DATE: 13944 19880111; FIRST REG.: FI EG 12766 19970825 |
| 2435025 | CA 2019 00032 | Denmark | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION AF GLYCOPYRROLAT, HERUNDER ALLE FARMACEUTISK ACCEPTABLE SALTE, ESTERE, ENANTIOMERER ELLER ANDRE DERIVATER DERAF, OG FORMOTEROL, HERUNDER ALLE FARMACEUTISK ACCEPTABLE SALTE, ESTERE, ENANTIOMERER ELLER ANDRE DERIVATER DERAF; REG. NO/DATE: EU/1/18/1339 20181220 |
| 0277829 | C300016 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: GANIRELIX, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; NATL REGISTRATION NO/DATE: EU/1/00/130/001-002 20000517 |
| 0480717 | 19/1999 | Austria | ⤷ Try a Trial | PRODUCT NAME: MONTELUKAST ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, INSBESONDERE MONTELUKASTNATRIUM; NAT. REGISTRATION NO/DATE: 1-22765 UND 1-22766 19981030; FIRST REGISTRATION: FI 12766 UND 12767 19970825 |
| 2435025 | 36/2019 | Austria | ⤷ Try a Trial | PRODUCT NAME: GLYCOPYRRONIUMBROMID / FORMOTEROL; REGISTRATION NO/DATE: EU/1/18/1339 (MITTEILUNG) 20181220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.