Neurocrine Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NEUROCRINE, and what generic alternatives to NEUROCRINE drugs are available?
NEUROCRINE has two approved drugs.
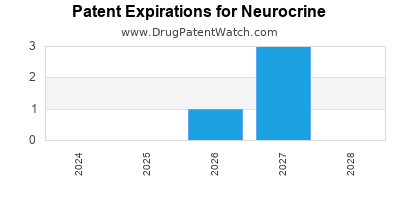
There are thirty-one US patents protecting NEUROCRINE drugs.
There are three hundred and one patent family members on NEUROCRINE drugs in forty-three countries and nineteen supplementary protection certificates in sixteen countries.
Drugs and US Patents for Neurocrine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-001 | Apr 11, 2017 | RX | Yes | No | 11,311,532 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-001 | Apr 11, 2017 | RX | Yes | No | 10,857,148 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-001 | Apr 11, 2017 | RX | Yes | No | 11,026,939 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-002 | Oct 4, 2017 | RX | Yes | Yes | 10,952,997 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Neurocrine Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Brazil | 112018008460 | ⤷ Try a Trial |
| Eurasian Patent Organization | 202090809 | ⤷ Try a Trial |
| Lithuania | 2481410 | ⤷ Try a Trial |
| Chile | 2018001089 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Neurocrine Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1907382 | PA2016036 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: OPIKAPONAS ARBA JO FARMACISKAI PRIIMTINOS DRUSKOS; REGISTRATION NO/DATE: EU/1/15/1066 20160624 |
| 1907382 | 93327 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: OPICAPONE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/15/1066 - ONGENTYS - OPICAPONE |
| 1907382 | C01907382/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: OPICAPON; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66547 26.04.2018 |
| 1907382 | 122016000095 | Germany | ⤷ Try a Trial | PRODUCT NAME: OPICAPON ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/15/1066 20160624 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.