Journey Company Profile
✉ Email this page to a colleague
What is the competitive landscape for JOURNEY, and what generic alternatives to JOURNEY drugs are available?
JOURNEY has eight approved drugs.
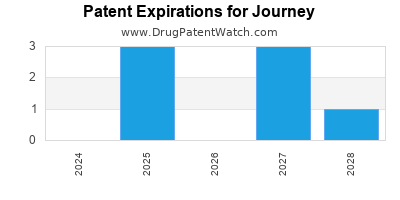
There are twenty-eight US patents protecting JOURNEY drugs.
There are seventy-one patent family members on JOURNEY drugs in seventeen countries and fifteen supplementary protection certificates in nine countries.
Summary for Journey
| International Patents: | 71 |
| US Patents: | 28 |
| Tradenames: | 6 |
| Ingredients: | 4 |
| NDAs: | 8 |
| Patent Litigation for Journey: | See patent lawsuits for Journey |
Drugs and US Patents for Journey
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Journey | AMZEEQ | minocycline hydrochloride | AEROSOL, FOAM;TOPICAL | 212379-001 | Oct 18, 2019 | RX | Yes | Yes | 10,398,641 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Journey | AMZEEQ | minocycline hydrochloride | AEROSOL, FOAM;TOPICAL | 212379-001 | Oct 18, 2019 | RX | Yes | Yes | 8,992,896 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Journey | EXELDERM | sulconazole nitrate | CREAM;TOPICAL | 018737-001 | Feb 28, 1989 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Journey | ZILXI | minocycline hydrochloride | AEROSOL, FOAM;TOPICAL | 213690-001 | May 28, 2020 | RX | Yes | Yes | 9,675,700 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Journey | QBREXZA | glycopyrronium tosylate | CLOTH;TOPICAL | 210361-001 | Jun 28, 2018 | RX | Yes | Yes | 10,543,192 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Journey | XIMINO | minocycline hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 201922-003 | Jul 11, 2012 | DISCN | No | No | 7,919,483 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Journey
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Journey | XIMINO | minocycline hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 201922-003 | Jul 11, 2012 | 5,908,838 | ⤷ Try a Trial |
| Journey | EXELDERM | sulconazole nitrate | SOLUTION;TOPICAL | 018738-001 | Aug 30, 1985 | 4,055,652 | ⤷ Try a Trial |
| Journey | XIMINO | minocycline hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 201922-001 | Jul 11, 2012 | 5,908,838 | ⤷ Try a Trial |
| Journey | QBREXZA | glycopyrronium tosylate | CLOTH;TOPICAL | 210361-001 | Jun 28, 2018 | 6,433,003 | ⤷ Try a Trial |
| Journey | XIMINO | minocycline hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 201922-005 | Jul 11, 2012 | 5,908,838 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Journey Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2013213047 | ⤷ Try a Trial |
| Australia | 2016269524 | ⤷ Try a Trial |
| Canada | 2902795 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2010017310 | ⤷ Try a Trial |
| Israel | 240684 | ⤷ Try a Trial |
| New Zealand | 710740 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Journey Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1267866 | CA 2014 00020 | Denmark | ⤷ Try a Trial | PRODUCT NAME: GLYCOPYRRONIUM ELLER ET SALT DERAF, ISAER BROMIDSALTET, I KOMBINATION MED INDACATEROL ELLER ET SALT DERAF, ISAER MALEATSALTET; REG. NO/DATE: EU/1/13/862 20130919 |
| 1267866 | 2013/014 | Ireland | ⤷ Try a Trial | PRODUCT NAME: GLYCOPYRRONIUM OR A SALT THEREOF; REGISTRATION NO/DATE: EU/1/12/788/001-006 20120928 |
| 2435024 | C02435024/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: BUDESONID, GLYCOPYRRONIUM UND FORMOTEROL; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 68388 24.11.2021 |
| 1267866 | 92166 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: GLYCOPYRRONIUM OU UN DE SES SELS |
| 2435024 | 15/2021 | Austria | ⤷ Try a Trial | PRODUCT NAME: FORMOTEROLFUMARAT-DIHYDRAT / GLYCOPYRRONIUMBROMID / BUDESONID; REGISTRATION NO/DATE: EU/1/20/1498 (MITTEILUNG) 20201210 |
| 1267866 | 2013C/023 | Belgium | ⤷ Try a Trial | PRODUCT NAME: GLYCOPYRRONIUM OU UN DE SES SELS; AUTHORISATION NUMBER AND DATE: EU/1/12/788/001 20121002 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.