Janssen Prods Company Profile
✉ Email this page to a colleague
What is the competitive landscape for JANSSEN PRODS, and what generic alternatives to JANSSEN PRODS drugs are available?
JANSSEN PRODS has seven approved drugs.
There are twenty-one US patents protecting JANSSEN PRODS drugs.
There are seven hundred and sixty-nine patent family members on JANSSEN PRODS drugs in sixty-four countries and two hundred and forty-eight supplementary protection certificates in nineteen countries.
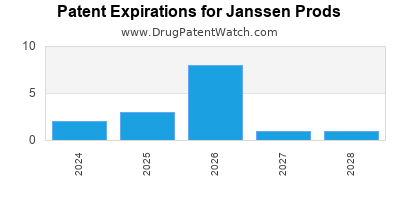
Drugs and US Patents for Janssen Prods
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Prods | PREZISTA | darunavir | SUSPENSION;ORAL | 202895-001 | Dec 16, 2011 | RX | Yes | Yes | 8,518,987*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 8,148,399 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 9,040,562 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-004 | Dec 18, 2008 | RX | Yes | No | 8,518,987*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | RX | Yes | Yes | 10,039,718 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Janssen Prods
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Prods | PREZISTA | darunavir | SUSPENSION;ORAL | 202895-001 | Dec 16, 2011 | 6,703,403*PED | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-005 | Dec 18, 2008 | 5,843,946*PED | ⤷ Try a Trial |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | 9,889,115 | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-006 | Nov 9, 2012 | RE43596*PED | ⤷ Try a Trial |
| Janssen Prods | PREZISTA | darunavir | TABLET;ORAL | 021976-003 | Oct 21, 2008 | 6,037,157*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for JANSSEN PRODS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 800 mg | ➤ Subscribe | 2013-05-14 |
International Patents for Janssen Prods Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Cyprus | 1118232 | ⤷ Try a Trial |
| European Patent Office | 1912999 | ⤷ Try a Trial |
| Eurasian Patent Organization | 014840 | ⤷ Try a Trial |
| Japan | 2014221845 | ⤷ Try a Trial |
| Norway | 2014032 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Janssen Prods Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1419152 | 132012902054377 | Italy | ⤷ Try a Trial | PRODUCT NAME: EMTRICITABINA/RILPIVIRINA CLORIDRATO/TENOFOVIR DISOPROXIL FUMARATO(EVIPLERA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/11/737/001-002, 20111128 |
| 1663240 | 222 6-2015 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: EMTRICITABIN/RILPIVIRINHYDROCHLORID; REGISTRATION NO/DATE: EU/1/11/737/001 - EU/1/11/737/002 20111128 |
| 1632232 | PA2016043 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRINO HIDROCHLORIDAS + TENOFOVIRO ALAFENAMIDAS; REGISTRATION NO/DATE: EU/1/16/1112 20160621 |
| 1912999 | 204 5025-2014 | Slovakia | ⤷ Try a Trial | FORMER OWNER(S): JANSSEN R&D IRELAND, IE; MEDIVIR AB, SE; DATUM ZAPISU DO REGISTRA: 20161206 |
| 1419152 | 1290017-1 | Sweden | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRIN OCH FARMACEUTISKT GODTAGBARA ADDITIONSSALTER DAERAV, INBEGRIPET VAETEKLORSYRASALTET AV RILPIVIRIN; REG. NO/DATE: EU/1/11/736/001 20111128 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.