Cipla Company Profile
✉ Email this page to a colleague
What is the competitive landscape for CIPLA, and when can generic versions of CIPLA drugs launch?
CIPLA has ninety-two approved drugs.
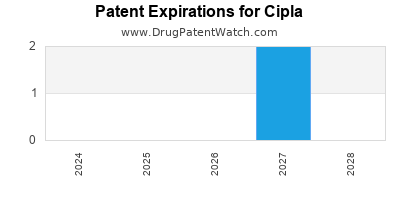
There are six US patents protecting CIPLA drugs. There are thirty-three tentative approvals on CIPLA drugs.
There are thirty patent family members on CIPLA drugs in twenty-one countries and four hundred and thirty-nine supplementary protection certificates in seventeen countries.
Summary for Cipla
| International Patents: | 30 |
| US Patents: | 6 |
| Tradenames: | 81 |
| Ingredients: | 81 |
| NDAs: | 92 |
| Patent Litigation for Cipla: | See patent lawsuits for Cipla |
| PTAB Cases with Cipla as petitioner: | See PTAB cases with Cipla as petitioner |
Drugs and US Patents for Cipla
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cipla | DIMETHYL FUMARATE | dimethyl fumarate | CAPSULE, DELAYED RELEASE;ORAL | 210305-002 | Sep 24, 2020 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla | AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE | amlodipine besylate; benazepril hydrochloride | CAPSULE;ORAL | 077215-002 | Dec 7, 2018 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Cipla | MEDROXYPROGESTERONE ACETATE | medroxyprogesterone acetate | INJECTABLE;INJECTION | 210335-001 | Jan 25, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Cipla | FENOFIBRATE | fenofibrate | TABLET;ORAL | 208709-001 | Dec 15, 2016 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla | CELECOXIB | celecoxib | CAPSULE;ORAL | 207446-004 | Sep 23, 2015 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla | ALENDRONATE SODIUM | alendronate sodium | TABLET;ORAL | 076768-001 | Aug 4, 2008 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Cipla Ltd | CARBOPLATIN | carboplatin | INJECTABLE;INJECTION | 077383-001 | Jan 27, 2006 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Cipla Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 101516370 | ⤷ Try a Trial |
| South Korea | 20100110297 | ⤷ Try a Trial |
| Japan | 4986310 | ⤷ Try a Trial |
| Eurasian Patent Organization | 017824 | ⤷ Try a Trial |
| Japan | 2011219498 | ⤷ Try a Trial |
| Brazil | PI0819319 | ⤷ Try a Trial |
| Poland | 2217610 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Cipla Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2666774 | CR 2020 00037 | Denmark | ⤷ Try a Trial | PRODUCT NAME: RELEBACTAM, OPTIONALLY IN THE FORM OF THE MONOHYDRATE, IMIPENEM AND CILASTATIN, OPTIONALLY IN THE FORM OF THE SODIUM SALT; REG. NO/DATE: EU/1/19/1420 20200217 |
| 0925294 | 07C0056 | France | ⤷ Try a Trial | PRODUCT NAME: LENALIDOMIDE; REGISTRATION NO/DATE: EU/1/07/391/001-004 20070618 |
| 1746976 | SPC/GB17/043 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: IRINOTECAN SUCROSOFATE SALT; REGISTERED: UK EU/1/16/1130 20161018 |
| 0613371 | SPC/GB02/033 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FORMOTEROL (OPTIONALLY IN THE FORM OF THE FREE BASE OR A PHYSIOLOGICALLY ACCEPTABLE SALT THEREOF, OR A SOLVATE OF SUCH FREE BASE OR SALT ESPECIALLY AS FORMOTEROL FUMARATE DIHYDRATE) AND BUDESONIDE; REGISTERED: SE SE16047, 16048 20000825; UK PL17901/0091 20010515; UK PL17901/0092 20010515 |
| 0503785 | C300486 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN OLMESARTANMEDOXOMIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, AMLODIPINEBESYLAAT AND HYDROCHLOORTHIAZIDE; NATL REGISTRATION NO/DATE: RVG 106667, RVG 106671-74, RVG 106682-86 20101221; FIRST REGISTRATION: DE 79810.00.00-79814.00.00 20101216 |
| 1663240 | 92854 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE RILPIVIRINE OU UNE FORME THERAPEUTIQUE EQUIVALENTE QUI EN DERIVE TELLE QUE PROTEGEE PAR LE BREVET DE BASE, TEL QU'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE RILPIVIRINE, INCLUANT LE SEL CHLORHYDRATE DE RILPIVIRINE, ET TENOFOVIR, EN PARTICULIER LE FUMARATE DE TENOFOVIR DISOPROXIL |
| 1632232 | SPC/GB17/007 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF RILPIVIRINE HYDROCHLORIDE AND TENOFOVIR ALAFENAMIDE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR TENOFOVIR ALAFENAMIDE FUMARATE; REGISTERED: UK EU/1/16/1112 (NI) 20160623; UK PLGB 11972/0019 20160623 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |