Bionpharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BIONPHARMA, and when can generic versions of BIONPHARMA drugs launch?
BIONPHARMA has sixty-six approved drugs.
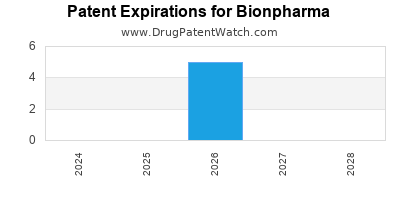
There are five US patents protecting BIONPHARMA drugs.
There are ten patent family members on BIONPHARMA drugs in thirteen countries and one hundred and seven supplementary protection certificates in fifteen countries.
Summary for Bionpharma
| International Patents: | 10 |
| US Patents: | 5 |
| Tradenames: | 61 |
| Ingredients: | 56 |
| NDAs: | 66 |
| Patent Litigation for Bionpharma: | See patent lawsuits for Bionpharma |
Drugs and US Patents for Bionpharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bionpharma | AMPHETAMINE SULFATE | amphetamine sulfate | TABLET;ORAL | 212919-002 | Nov 22, 2019 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | LOPERAMIDE HYDROCHLORIDE | loperamide hydrochloride | CAPSULE;ORAL | 021855-002 | Aug 4, 2005 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bionpharma | GRANISETRON HYDROCHLORIDE PRESERVATIVE FREE | granisetron hydrochloride | INJECTABLE;INJECTION | 078863-002 | Jun 30, 2008 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | METHYLPHENIDATE HYDROCHLORIDE | methylphenidate hydrochloride | TABLET;ORAL | 209753-002 | Mar 2, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | CLOBAZAM | clobazam | TABLET;ORAL | 208825-002 | Oct 22, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bionpharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bionpharma | MIDOL LIQUID GELS | ibuprofen | CAPSULE;ORAL | 021472-001 | Oct 18, 2002 | 6,251,426 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Bionpharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Hungary | E030784 | ⤷ Try a Trial |
| Cyprus | 1118321 | ⤷ Try a Trial |
| Slovenia | 1863458 | ⤷ Try a Trial |
| Lithuania | 1863458 | ⤷ Try a Trial |
| Spain | 2606767 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bionpharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0994863 | SPC/GB07/041 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: CRYSTAL MODIFICATION A OF RUFINAMIDE; REGISTRATION NO/DATE: EU/1/06/378/001 - 015 |
| 0716606 | C00716606/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: SEVELAMER; REGISTRATION NUMBER/DATE: SWISSMEDIC 56297 10.02.2004 |
| 0123238 | SPC/GB95/004 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ATOVAQUONE OPTIONALLY IN THE FORM OF A PHYSIOLOGICALLY ACCEPTABLE SALT; REGISTERED: LU 0458/94/08/0741 19940803; UK 0003/0337 19940823 |
| 1534313 | CR 2015 00072 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PHENYLEPHRIN, HERUNDER PHENYLEPHRINHYDROCHLORID OG KETOROLAC, HERUNDER KETOROLACTROMETAMOL; REG. NO/DATE: EU/1/15/1018 20150730 |
| 1534313 | 92923 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: UNE SOLUTION D'IRRIGATION OCULAIRE COMPRENANT DE LA PHENYLEPHRINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI ET DU KETOROLAC OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; FIRST REGISTRATION: 20150730 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.