Bausch And Lomb Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BAUSCH AND LOMB INC, and when can generic versions of BAUSCH AND LOMB INC drugs launch?
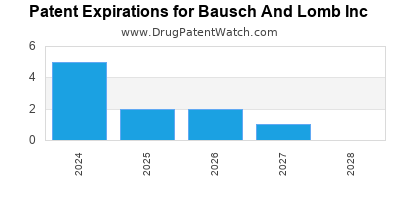
BAUSCH AND LOMB INC has nineteen approved drugs.
There are thirty-two US patents protecting BAUSCH AND LOMB INC drugs.
There are three hundred and seventy-four patent family members on BAUSCH AND LOMB INC drugs in thirty-one countries and forty-one supplementary protection certificates in fourteen countries.
Summary for Bausch And Lomb Inc
| International Patents: | 374 |
| US Patents: | 32 |
| Tradenames: | 19 |
| Ingredients: | 15 |
| NDAs: | 19 |
| Drug Master File Entries: | 1 |
Drugs and US Patents for Bausch And Lomb Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch And Lomb Inc | XIPERE | triamcinolone acetonide | SUSPENSION;INJECTION | 211950-001 | Oct 22, 2021 | RX | Yes | Yes | 9,937,075 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bausch And Lomb Inc | LUMIFY | brimonidine tartrate | SOLUTION/DROPS;OPHTHALMIC | 208144-001 | Dec 22, 2017 | OTC | Yes | Yes | 8,293,742 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch And Lomb Inc | LUMIFY | brimonidine tartrate | SOLUTION/DROPS;OPHTHALMIC | 208144-001 | Dec 22, 2017 | OTC | Yes | Yes | 11,833,245 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bausch And Lomb Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch And Lomb Inc | BEPREVE | bepotastine besilate | SOLUTION/DROPS;OPHTHALMIC | 022288-001 | Sep 8, 2009 | 6,780,877 | ⤷ Try a Trial |
| Bausch And Lomb Inc | MACUGEN | pegaptanib sodium | INJECTABLE;INTRAVITREAL | 021756-001 | Dec 17, 2004 | 6,051,698 | ⤷ Try a Trial |
| Bausch And Lomb Inc | TIMOPTIC | timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 018086-001 | Approved Prior to Jan 1, 1982 | 3,655,663 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAUSCH AND LOMB INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Ophthalmic Solution | 1.5% | ➤ Subscribe | 2013-09-09 |
International Patents for Bausch And Lomb Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2782817 | ⤷ Try a Trial |
| Japan | 2018111704 | ⤷ Try a Trial |
| South Korea | 102115111 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bausch And Lomb Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0509752 | C990041 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DORZOLAMIDE, DESGEWENST IN DE VORM VAN EEN OFTALMOLOGISCH AAN- VAARDBAAR ZOUT, EN TIMOLOL, DESGEWENST IN DE VORM VAN EEN OFTAL -MOLOGISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER DORZOLLAMIDEHY- DROCHLORIDE EN TIMOLOLMALEAAT, EEN EN ANDER ZODANIG DAT 0,05; NATL REGISTRATION NO/DATE: VG 22871 19980805; FIRST REGISTRATION: DK 19045 19980306 |
| 0509752 | 49/1999 | Austria | ⤷ Try a Trial | PRODUCT NAME: DORZOLAMID ODER EIN OPHTHALMOLOGISCH ANNEHMBARES SALZ DAVON, VORZUGSWEISE DORZOLAMIDHYDROCHLORID, UND TIMOLOL ODER EIN OPHTHALMOLOGISCH ANNEHMBARES SALZ DAVON, VORZUGSWEISE TIMOLOLMALEAT; NAT. REGISTRATION NO/DATE: 1-22701, 1-22702 19980828; FIRST REGISTRATION: DK 9794 19980306 |
| 3043773 | 301154 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: MOMETASON OF EEN ZOUT DAARVAN EN OLOPATADINE OF EEN ZOUT DAARVAN; NATIONAL REGISTRATION NO/DATE: RVG 126186 20211014; FIRST REGISTRATION: AT 140638 20210426 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.