Astrazeneca Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA, and when can generic versions of ASTRAZENECA drugs launch?
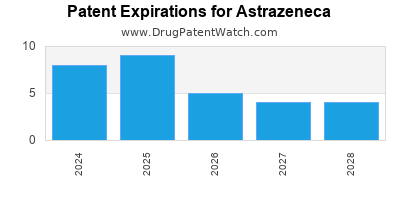
ASTRAZENECA has ninety-three approved drugs.
There are one hundred and twenty-four US patents protecting ASTRAZENECA drugs. There is one tentative approval on ASTRAZENECA drugs.
There are two thousand one hundred and ninety-two patent family members on ASTRAZENECA drugs in seventy countries and three hundred and forty-six supplementary protection certificates in twenty countries.
Summary for Astrazeneca
| International Patents: | 2192 |
| US Patents: | 124 |
| Tradenames: | 66 |
| Ingredients: | 60 |
| NDAs: | 93 |
| Patent Litigation for Astrazeneca: | See patent lawsuits for Astrazeneca |
| PTAB Cases with Astrazeneca as patent owner: | See PTAB cases with Astrazeneca as patent owner |
Drugs and US Patents for Astrazeneca
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | RX | Yes | Yes | 9,238,076 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | RX | Yes | No | 8,501,698 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca | BEVESPI AEROSPHERE | formoterol fumarate; glycopyrrolate | AEROSOL, METERED;INHALATION | 208294-001 | Apr 25, 2016 | RX | Yes | Yes | 8,324,266 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-002 | Aug 17, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Astrazeneca
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | NEXIUM | esomeprazole magnesium | CAPSULE, DELAYED REL PELLETS;ORAL | 021153-002 | Feb 20, 2001 | 4,508,905 | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | RE44186 | ⤷ Try a Trial |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-001 | Mar 16, 2005 | 7,407,934 | ⤷ Try a Trial |
| Astrazeneca | ELAVIL | amitriptyline hydrochloride | TABLET;ORAL | 012703-007 | Approved Prior to Jan 1, 1982 | 3,428,735 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTRAZENECA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 25 mg | ➤ Subscribe | 2005-08-12 |
| ➤ Subscribe | Delayed-release | 20 mg and 40 mg | ➤ Subscribe | 2005-08-05 |
| ➤ Subscribe | Tablets | 100 mg, 200 mg and 300 mg | ➤ Subscribe | 2006-02-21 |
| ➤ Subscribe | For Injection | 20 mg/vial and 40 mg/vial | ➤ Subscribe | 2009-11-23 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Inhalation Suspension | 0.25 mg/2 mL and 0.5 mg/2 mL | ➤ Subscribe | 2005-09-15 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Delayed-release Capsules | 20 mg | ➤ Subscribe | 2007-03-19 |
| ➤ Subscribe | Extended-release Tablets | 400 mg | ➤ Subscribe | 2008-06-18 |
| ➤ Subscribe | Tablets | 200 mg and 300 mg | ➤ Subscribe | 2008-06-12 |
| ➤ Subscribe | Tablets | 90 mg | ➤ Subscribe | 2015-07-20 |
| ➤ Subscribe | Tablets | 500 mcg | ➤ Subscribe | 2015-03-02 |
| ➤ Subscribe | Nasal Spray | 0.032 mg (32 mcg)/spray | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | Tablets | 50 mg, 150 mg and 400 mg | ➤ Subscribe | 2007-02-12 |
| ➤ Subscribe | Delayed-release for Oral Suspension | 2.5 mg and 5 mg | ➤ Subscribe | 2018-09-24 |
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
| ➤ Subscribe | Injection | 50 mg/mL, 2.5 mL and 5 mL syringe | ➤ Subscribe | 2009-10-01 |
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Inhalation Suspension | 1 mg/2 mL | ➤ Subscribe | 2010-05-28 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | Extended-release Tablets | 150 mg | ➤ Subscribe | 2008-11-17 |
| ➤ Subscribe | Extended-release Tablets | 50 mg | ➤ Subscribe | 2008-10-17 |
| ➤ Subscribe | Tablets | 60 mg | ➤ Subscribe | 2015-09-30 |
International Patents for Astrazeneca Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| South Korea | 20120015334 | ⤷ Try a Trial |
| Taiwan | I519528 | ⤷ Try a Trial |
| Colombia | 6210728 | ⤷ Try a Trial |
| Israel | 180096 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Astrazeneca Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0957929 | 06C0021 | France | ⤷ Try a Trial | PRODUCT NAME: PEGAPTANIB SODIUM; REGISTRATION NO/DATE: EU/1/05/325/001 20060131 |
| 0861666 | 07C0006 | France | ⤷ Try a Trial | PRODUCT NAME: PIOGLITAZONE/METFORMINE ET LEURS SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE IN FRANCE: EU/1/06/354/001 DU 20060728; REGISTRATION NO/DATE AT EEC: EU/1/06/354/001 DU 20060728 |
| 1506211 | 122014000070 | Germany | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON DAPAGLIFLOZIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON UND METFORMIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON, WIE DURCH DAS GRUNDPATENT GESCHUETZT; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| 1412357 | C 2008 016 | Romania | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN OPTIONAL SUB FORMA DE SARE ACCEPTABILAFARMACEUTIC IN SPECIAL MONOFOSFAT + METFORMIN OPTIONAL SUB FORMA DE SARE ACCEPTABILA FARMACEUTIC IN SPECIALCLORHIDRAT; NATIONAL AUTHORISATION NUMBER: RO EU/1/08/455/001 - RO EU/1/08/455/014; DATE OF NATIONAL AUTHORISATION: 20080716; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): CH 58450 01, CH 58450 02, CH 58450 03; DATE OF FIRST AUTHORISATION IN EEA: 20080408 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.