Am Regent Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AM REGENT, and what generic alternatives to AM REGENT drugs are available?
AM REGENT has seventy-seven approved drugs.
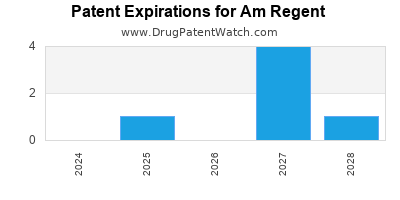
There are seven US patents protecting AM REGENT drugs.
There are sixty-seven patent family members on AM REGENT drugs in thirty-two countries and one hundred and forty-three supplementary protection certificates in fifteen countries.
Drugs and US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | GLYCOPYRROLATE | glycopyrrolate | INJECTABLE;INJECTION | 089335-001 | Jul 23, 1986 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | CLONIDINE HYDROCHLORIDE | clonidine hydrochloride | INJECTABLE;INJECTION | 091104-001 | Oct 8, 2009 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 9,376,505 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,291,645 | ⤷ Try a Trial |
| Am Regent | DEXFERRUM | ferric oxyhydroxide | INJECTABLE;INJECTION | 040024-001 | Feb 23, 1996 | 5,624,668 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Am Regent Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Mexico | PA05004300 | ⤷ Try a Trial |
| European Patent Office | 1973549 | ⤷ Try a Trial |
| Canada | 2635894 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Am Regent Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2957286 | 6/2019 | Austria | ⤷ Try a Trial | PRODUCT NAME: PATIROMER UND SEINE CALCIUM-SALZE; REGISTRATION NO/DATE: EU/1/17/1179 (MITTEILUNG) 20170721 |
| 0503785 | CA 2011 00026 | Denmark | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF OLMESARTAN MEDOXOMIL, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, AND AMLODIPINE BESYLATE AND HYDROCHLOROTHIAZIDE; NAT. REG. NO/DATE: 46260-46269 (DK) 20110323; FIRST REG. NO/DATE: DE 79810.00.00 20101216 |
| 0145340 | 99C0005 | Belgium | ⤷ Try a Trial | PRODUCT NAME: FOSPHENYTOIN DISODIUM; NAT. REGISTRATION NO/DATE: NL 23 613 19980806; FIRST REGISTRATION: GB - PL 000 19/0157 19980204 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.