Naproxen sodium - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for naproxen sodium and what is the scope of freedom to operate?
Naproxen sodium
is the generic ingredient in nine branded drugs marketed by Bionpharma, Catalent, Patheon Softgels, Puracap Pharm Llc, Strides Pharma, Twi Pharms, Actavis Labs Fl Inc, Bayer, Atnahs Pharma Us, Able, Amneal Pharms Ny, Aurobindo Pharma Ltd, Contract Pharmacal, Dr Reddys Labs Inc, Dr Reddys Labs Ltd, Glenmark Pharms Ltd, Granules India, Hamilton Pharms, Hetero Labs Ltd V, Hikma, Ivax Sub Teva Pharms, Lnk Intl Inc, Marksans Pharma, Mylan, Novelgenix Theraps, Perrigo, Pld Acquisitions Llc, Pliva, Purepac Pharm, Roxane, Sandoz, Sciegen Pharms Inc, Sun Pharm Inds Ltd, Teva, Teva Pharms, Watson Labs, Yichang Humanwell, Aurobindo Pharma, Rising, Sun Pharm, and Currax, and is included in fifty-one NDAs. There are six patents protecting this compound. Additional information is available in the individual branded drug profile pages.Naproxen sodium has fifteen patent family members in thirteen countries.
There are twenty-two drug master file entries for naproxen sodium. One hundred and thirty-five suppliers are listed for this compound.
Summary for naproxen sodium
| International Patents: | 15 |
| US Patents: | 6 |
| Tradenames: | 9 |
| Applicants: | 41 |
| NDAs: | 51 |
| Drug Master File Entries: | 22 |
| Finished Product Suppliers / Packagers: | 135 |
| Raw Ingredient (Bulk) Api Vendors: | 93 |
| Clinical Trials: | 100 |
| Patent Applications: | 7,012 |
| Formulation / Manufacturing: | see details |
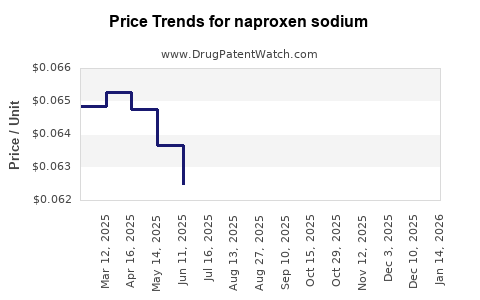
| Drug Prices: | Drug price trends for naproxen sodium |
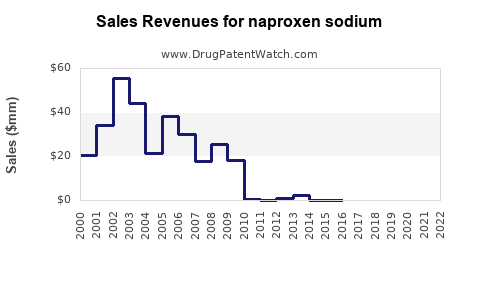
| Drug Sales Revenues: | Drug sales revenues for naproxen sodium |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for naproxen sodium |
| What excipients (inactive ingredients) are in naproxen sodium? | naproxen sodium excipients list |
| DailyMed Link: | naproxen sodium at DailyMed |
Recent Clinical Trials for naproxen sodium
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Johnson & Johnson Consumer Inc. (J&JCI) | Phase 1 |
| Johnson & Johnson Consumer Inc. (J&JCI) | Phase 3 |
| Ciusss de L'Est de l'Île de Montréal | N/A |
Pharmacology for naproxen sodium
| Drug Class | Nonsteroidal Anti-inflammatory Drug |
| Mechanism of Action | Cyclooxygenase Inhibitors |
Paragraph IV (Patent) Challenges for NAPROXEN SODIUM
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NAPROXEN SODIUM | Capsules | naproxen sodium | 200 mg | 021920 | 1 | 2017-11-15 |
US Patents and Regulatory Information for naproxen sodium
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074142-001 | Dec 21, 1993 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novelgenix Theraps | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 207612-001 | Nov 16, 2018 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Watson Labs | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074195-001 | Dec 21, 1993 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for naproxen sodium
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Atnahs Pharma Us | ANAPROX DS | naproxen sodium | TABLET;ORAL | 018164-003 | Sep 30, 1987 | ⤷ Try a Trial | ⤷ Try a Trial |
| Atnahs Pharma Us | ANAPROX DS | naproxen sodium | TABLET;ORAL | 018164-003 | Sep 30, 1987 | ⤷ Try a Trial | ⤷ Try a Trial |
| Twi Pharms | NAPRELAN | naproxen sodium | TABLET, EXTENDED RELEASE;ORAL | 020353-003 | Jan 5, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for naproxen sodium
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2600023 | SYSTEME DE SOLVANT DESTINE A AMELIORER LA SOLUBILITE DES AGENTS PHARMACEUTIQUES (SOLVENT SYSTEM FOR ENHANCING THE SOLUBILITY OF PHARMACEUTICAL AGENTS) | ⤷ Try a Trial |
| Spain | 2606767 | ⤷ Try a Trial | |
| Mexico | 2007011039 | SISTEMA DE SOLVENTE PARA AUMENTAR LA SOLUBILIDAD DE AGENTES FARMACEUTICOS. (SOLVENT SYSTEM FOR ENHANCING THE SOLUBILITY OF PHARMACEUTICAL AGENTS.) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for naproxen sodium
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0984957 | PA2011005 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: NAPROXENUM + ESOMEPRAZOLUM; REGISTRATION NO/DATE: LT/1/10/2302/001-LT/1/10/2302/012 20110126 |
| 1411900 | 18/2011 | Austria | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN UND ESOMEPRAZOL SOWIE DEREN PHARMAZEUTISCH ANNEHMBARE SALZE; NAT. REGISTRATION NO/DATE: 1-29937 20110105; FIRST REGISTRATION: GB PL 17901/0263-0001 20101105 |
| 0984957 | PA2011005,C0984957 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: NAPROXENUM + ESOMEPRAZOLUM; REGISTRATION NO/DATE: LT/1/10/2302/001-LT/1/10/2302/012 20110126 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.