Takeda Pharms Usa Company Profile
✉ Email this page to a colleague
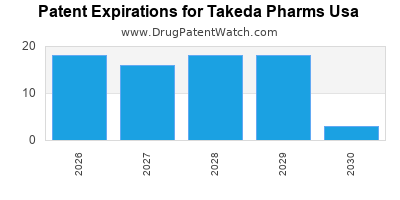
What is the competitive landscape for TAKEDA PHARMS USA, and when can generic versions of TAKEDA PHARMS USA drugs launch?
TAKEDA PHARMS USA has forty approved drugs.
There are one hundred and six US patents protecting TAKEDA PHARMS USA drugs.
There are one thousand four hundred and twenty-eight patent family members on TAKEDA PHARMS USA drugs in fifty-nine countries and one hundred and eighty-eight supplementary protection certificates in nineteen countries.
Summary for Takeda Pharms Usa
| International Patents: | 1428 |
| US Patents: | 106 |
| Tradenames: | 43 |
| Ingredients: | 33 |
| NDAs: | 40 |
Drugs and US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | OMONTYS | peginesatide acetate | SOLUTION;INTRAVENOUS, SUBCUTANEOUS | 202799-008 | Mar 27, 2012 | DISCN | No | No | 7,550,433 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Takeda Pharms Usa | FOSRENOL | lanthanum carbonate | TABLET, CHEWABLE;ORAL | 021468-004 | Nov 23, 2005 | AB | RX | Yes | Yes | 7,465,465 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | OSENI | alogliptin benzoate; pioglitazone hydrochloride | TABLET;ORAL | 022426-005 | Jan 25, 2013 | RX | Yes | No | 8,288,539 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Takeda Pharms Usa | EXKIVITY | mobocertinib succinate | CAPSULE;ORAL | 215310-001 | Sep 15, 2021 | RX | Yes | Yes | 9,796,712 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-003 | Sep 30, 2013 | DISCN | Yes | No | 9,125,910*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Takeda Pharms Usa | CARBATROL | carbamazepine | CAPSULE, EXTENDED RELEASE;ORAL | 020712-002 | Sep 30, 1997 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | PREVACID | lansoprazole | TABLET, ORALLY DISINTEGRATING, DELAYED RELEASE;ORAL | 021428-001 | Aug 30, 2002 | 7,431,942*PED | ⤷ Try a Trial |
| Takeda Pharms Usa | KAZANO | alogliptin benzoate; metformin hydrochloride | TABLET;ORAL | 203414-002 | Jan 25, 2013 | 6,890,898 | ⤷ Try a Trial |
| Takeda Pharms Usa | DUETACT | glimepiride; pioglitazone hydrochloride | TABLET;ORAL | 021925-001 | Jul 28, 2006 | 6,303,640 | ⤷ Try a Trial |
| Takeda Pharms Usa | ACTOS | pioglitazone hydrochloride | TABLET;ORAL | 021073-001 | Jul 15, 1999 | 6,329,404 | ⤷ Try a Trial |
| Takeda Pharms Usa | VELCADE | bortezomib | INJECTABLE;INTRAVENOUS, SUBCUTANEOUS | 021602-001 | May 13, 2003 | 6,617,317 | ⤷ Try a Trial |
| Takeda Pharms Usa | ACTOPLUS MET XR | metformin hydrochloride; pioglitazone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022024-001 | May 12, 2009 | 6,495,162 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TAKEDA PHARMS USA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Capsules | 37.5 mg and50 mg | ➤ Subscribe | 2017-08-03 |
| ➤ Subscribe | Tablets | 40 mg and 80 mg | ➤ Subscribe | 2013-02-13 |
| ➤ Subscribe | Capsules | 20 mg, 30 mg, 40 mg, 50 mg, 60 mg and 70 mg | ➤ Subscribe | 2011-02-23 |
| ➤ Subscribe | Injection | 10 mg/mL | ➤ Subscribe | 2015-08-25 |
| ➤ Subscribe | Oral Powder | 750 mg and 1000 mg | ➤ Subscribe | 2015-11-25 |
| ➤ Subscribe | Extended-release Tablets | 15 mg/1000 mg and 30 mg/1000 mg | ➤ Subscribe | 2011-09-23 |
| ➤ Subscribe | Tablets | 15 mg/500 mg and 15 mg/850 mg | ➤ Subscribe | 2008-03-06 |
| ➤ Subscribe | Delayed-release Pellets/Capsul | 15 mg and 30 mg | ➤ Subscribe | 2005-12-05 |
| ➤ Subscribe | Tablets | 30 mg/2 mg and 30 mg/4 mg | ➤ Subscribe | 2009-12-22 |
| ➤ Subscribe | Capsule | 60 mg | ➤ Subscribe | 2010-08-25 |
| ➤ Subscribe | Tablets | 6.25 mg, 12.5 mg and 25 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Extended-release Capsules | 100 mg and 200 mg | ➤ Subscribe | 2006-02-02 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 15 mgand 20 mg | ➤ Subscribe | 2017-10-02 |
| ➤ Subscribe | Extended-release Capsules | 12.5 mg and 25 mg | ➤ Subscribe | 2017-08-07 |
| ➤ Subscribe | For Injection | 3.5 mg/vial | ➤ Subscribe | 2008-11-20 |
| ➤ Subscribe | Chewable Tablet | 500 mg, 750 mg and 1000 mg | ➤ Subscribe | 2008-10-27 |
| ➤ Subscribe | Delayed-release Tablets | 1.2 g | ➤ Subscribe | 2009-12-16 |
| ➤ Subscribe | Extended-release Tablets | 1 mg, 2 mg, 3 mg and 4 mg | ➤ Subscribe | 2009-12-29 |
| ➤ Subscribe | Tablets | 0.6 mg | ➤ Subscribe | 2011-12-23 |
| ➤ Subscribe | Delayed-release Orally Disinte | 15 mg and 30 mg | ➤ Subscribe | 2006-12-27 |
| ➤ Subscribe | Tablets | 12.5 mg/500 mg and 12.5 mg/1000 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Delayed-release Capsule | 30 mg | ➤ Subscribe | 2010-11-30 |
| ➤ Subscribe | Tablets | 8 mg | ➤ Subscribe | 2009-07-22 |
International Patents for Takeda Pharms Usa Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| South Africa | 200707137 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2009080464 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2014093583 | ⤷ Try a Trial |
| Eurasian Patent Organization | 200600414 | ⤷ Try a Trial |
| Portugal | 2107905 | ⤷ Try a Trial |
| Austria | 438650 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Takeda Pharms Usa Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261586 | 2012C/016 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE PRODUITS DE SAXAGLIPTINE ET DE METFORMINE AINSI QUE TOUT SELS PHARMACEUTIQUEMENT ACCEPTABLES, DONT LES SELS DE CHLORHYDRATE DE SAXAGLIPTINE ET DE METFORMINE; AUTHORISATION NUMBER AND DATE: EU/1/11/731/001 20111128 |
| 2498758 | 132020000000034 | Italy | ⤷ Try a Trial | PRODUCT NAME: METFORMINA O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE; SAXAGLIPTIN O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE; DAPAGLIFLOZIN O UN SUO SOLVATO FARMACEUTICAMENTE ACCETTABILE.(QTRILMET); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/19/1401, 20191113 |
| 1506211 | C300677 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN METFORMINE OF EEN FARMACEITISCH AANVAARDBAAR ZOUT DAARVAN, ZOALS BESCHERMD DOOR HET BASISOCTROOI EP 1506211B1; REGISTRATION NO/DATE: EU/1/13/900 20140121 |
| 1412357 | DO 77; 5006-2008 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN A METFORMIN; REGISTRATION NO/DATE: EU/1/08/455/001-014 20080716 |
| 1436271 | 2014C/036 | Belgium | ⤷ Try a Trial | PRODUCT NAME: VORTIOXETINE; AUTHORISATION NUMBER AND DATE: EU/1/13/891 20131220 |
| 2300013 | 31/2019 | Austria | ⤷ Try a Trial | PRODUCT NAME: BRIGATINIB ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/18/1264 (MITTEILUNG) 20181126 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.