Takeda Pharms Usa Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TAKEDA PHARMS USA, and when can generic versions of TAKEDA PHARMS USA drugs launch?
TAKEDA PHARMS USA has forty approved drugs.
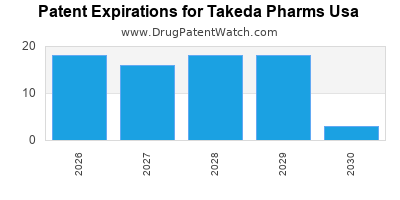
There are one hundred and six US patents protecting TAKEDA PHARMS USA drugs.
There are one thousand four hundred and twenty-seven patent family members on TAKEDA PHARMS USA drugs in fifty-nine countries and one hundred and eighty-eight supplementary protection certificates in nineteen countries.
Summary for Takeda Pharms Usa
| International Patents: | 1427 |
| US Patents: | 106 |
| Tradenames: | 43 |
| Ingredients: | 33 |
| NDAs: | 40 |
Drugs and US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | OMONTYS PRESERVATIVE FREE | peginesatide acetate | SOLUTION;INTRAVENOUS, SUBCUTANEOUS | 202799-003 | Mar 27, 2012 | DISCN | No | No | 7,919,118 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | VYVANSE | lisdexamfetamine dimesylate | TABLET, CHEWABLE;ORAL | 208510-001 | Jan 28, 2017 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Takeda Pharms Usa | OSENI | alogliptin benzoate; pioglitazone hydrochloride | TABLET;ORAL | 022426-005 | Jan 25, 2013 | RX | Yes | No | 8,173,663 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Takeda Pharms Usa | OSENI | alogliptin benzoate; pioglitazone hydrochloride | TABLET;ORAL | 022426-004 | Jan 25, 2013 | DISCN | Yes | No | 7,807,689 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | MOTEGRITY | prucalopride succinate | TABLET;ORAL | 210166-001 | Dec 14, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Takeda Pharms Usa | ICLUSIG | ponatinib hydrochloride | TABLET;ORAL | 203469-002 | Dec 14, 2012 | RX | Yes | Yes | 9,029,533 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | MYDAYIS | amphetamine aspartate; amphetamine sulfate; dextroamphetamine saccharate; dextroamphetamine sulfate | CAPSULE, EXTENDED RELEASE;ORAL | 022063-004 | Jun 20, 2017 | RE42096 | ⤷ Try a Trial |
| Takeda Pharms Usa | VELCADE | bortezomib | INJECTABLE;INTRAVENOUS, SUBCUTANEOUS | 021602-001 | May 13, 2003 | 7,119,080 | ⤷ Try a Trial |
| Takeda Pharms Usa | OSENI | alogliptin benzoate; pioglitazone hydrochloride | TABLET;ORAL | 022426-004 | Jan 25, 2013 | 6,329,404 | ⤷ Try a Trial |
| Takeda Pharms Usa | ACTOS | pioglitazone hydrochloride | TABLET;ORAL | 021073-001 | Jul 15, 1999 | 6,211,205 | ⤷ Try a Trial |
| Takeda Pharms Usa | INTUNIV | guanfacine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022037-003 | Sep 2, 2009 | 6,287,599*PED | ⤷ Try a Trial |
| Takeda Pharms Usa | ULORIC | febuxostat | TABLET;ORAL | 021856-002 | Feb 13, 2009 | 5,614,520 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TAKEDA PHARMS USA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Capsule | 30 mg | ➤ Subscribe | 2010-11-30 |
| ➤ Subscribe | Tablets | 8 mg | ➤ Subscribe | 2009-07-22 |
| ➤ Subscribe | Extended-release Capsules | 37.5 mg and50 mg | ➤ Subscribe | 2017-08-03 |
| ➤ Subscribe | Tablets | 40 mg and 80 mg | ➤ Subscribe | 2013-02-13 |
| ➤ Subscribe | Capsules | 20 mg, 30 mg, 40 mg, 50 mg, 60 mg and 70 mg | ➤ Subscribe | 2011-02-23 |
| ➤ Subscribe | Injection | 10 mg/mL | ➤ Subscribe | 2015-08-25 |
| ➤ Subscribe | Oral Powder | 750 mg and 1000 mg | ➤ Subscribe | 2015-11-25 |
| ➤ Subscribe | Extended-release Tablets | 15 mg/1000 mg and 30 mg/1000 mg | ➤ Subscribe | 2011-09-23 |
| ➤ Subscribe | Tablets | 15 mg/500 mg and 15 mg/850 mg | ➤ Subscribe | 2008-03-06 |
| ➤ Subscribe | Delayed-release Pellets/Capsul | 15 mg and 30 mg | ➤ Subscribe | 2005-12-05 |
| ➤ Subscribe | Tablets | 30 mg/2 mg and 30 mg/4 mg | ➤ Subscribe | 2009-12-22 |
| ➤ Subscribe | Capsule | 60 mg | ➤ Subscribe | 2010-08-25 |
| ➤ Subscribe | Tablets | 6.25 mg, 12.5 mg and 25 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Extended-release Capsules | 100 mg and 200 mg | ➤ Subscribe | 2006-02-02 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 15 mgand 20 mg | ➤ Subscribe | 2017-10-02 |
| ➤ Subscribe | Extended-release Capsules | 12.5 mg and 25 mg | ➤ Subscribe | 2017-08-07 |
| ➤ Subscribe | For Injection | 3.5 mg/vial | ➤ Subscribe | 2008-11-20 |
| ➤ Subscribe | Chewable Tablet | 500 mg, 750 mg and 1000 mg | ➤ Subscribe | 2008-10-27 |
| ➤ Subscribe | Delayed-release Tablets | 1.2 g | ➤ Subscribe | 2009-12-16 |
| ➤ Subscribe | Extended-release Tablets | 1 mg, 2 mg, 3 mg and 4 mg | ➤ Subscribe | 2009-12-29 |
| ➤ Subscribe | Tablets | 0.6 mg | ➤ Subscribe | 2011-12-23 |
| ➤ Subscribe | Delayed-release Orally Disinte | 15 mg and 30 mg | ➤ Subscribe | 2006-12-27 |
| ➤ Subscribe | Tablets | 12.5 mg/500 mg and 12.5 mg/1000 mg | ➤ Subscribe | 2017-01-25 |
International Patents for Takeda Pharms Usa Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 1629007 | ⤷ Try a Trial |
| Japan | 6239951 | ⤷ Try a Trial |
| Poland | 2300013 | ⤷ Try a Trial |
| South Korea | 20040101284 | ⤷ Try a Trial |
| Singapore | 10201405001X | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006050244 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Takeda Pharms Usa Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1973545 | C 2013 039 | Romania | ⤷ Try a Trial | PRODUCT NAME: PONATINIB SI SARURILE SALE ACCEPTABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/13/839/001, EU/1/13/839/002, EU/1/13/839/003, EU/1/13/839/004; DATE OF NATIONAL AUTHORISATION: 20130701; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/839/001, EU/1/13/839/002, EU/1/13/839/003, EU/1/13/839/004; DATE OF FIRST AUTHORISATION IN EEA: 20130701 |
| 2435024 | 301102 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN FORMOTEROL (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN), GLYCOPYRROLAAT (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN) EN BUDESONIDE (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| 1436271 | C01436271/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: VORTIOXETINE; REGISTRATION NO/DATE: SWISSMEDIC 65937 09.06.2016 |
| 2300013 | LUC00120 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: BRIGATINIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/18/1264 20181126 |
| 2178888 | CR 2017 00014 | Denmark | ⤷ Try a Trial | PRODUCT NAME: IXAZOMIB OG FARMACEUTISK ACCEPTABLE SALTE OG ESTERE DERAF, HERUNDER IXAZOMIBCITRAT; REG. NO/DATE: EU/1/16/1094 20161123 |
| 1506211 | C 2014 029 | Romania | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE DE DAPAGLIFLOZIN SAU O SARE ACCEPTABILAFARMACEUTIC A ACESTUIA SI METFORMINA SAU O SARE ACCEPTABILA FARMACEUTIC A ACESTEIA; NATIONAL AUTHORISATION NUMBER: EU/1/13/900; DATE OF NATIONAL AUTHORISATION: 20140116; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/900; DATE OF FIRST AUTHORISATION IN EEA: 20140116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.