Taro Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TARO, and what generic alternatives to TARO drugs are available?
TARO has two hundred and fifty-five approved drugs.
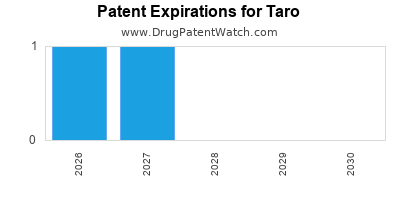
There are eight US patents protecting TARO drugs. There are four tentative approvals on TARO drugs.
There are thirty-one patent family members on TARO drugs in twelve countries and one hundred and eighty-seven supplementary protection certificates in fourteen countries.
Summary for Taro
| International Patents: | 31 |
| US Patents: | 8 |
| Tradenames: | 134 |
| Ingredients: | 117 |
| NDAs: | 255 |
| Patent Litigation for Taro: | See patent lawsuits for Taro |
| PTAB Cases with Taro as petitioner: | See PTAB cases with Taro as petitioner |
Drugs and US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 208776-001 | May 25, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | OINTMENT;TOPICAL | 040037-001 | Sep 30, 1994 | AT | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro Pharms North | PSORCON | diflorasone diacetate | CREAM;TOPICAL | 020205-001 | Nov 20, 1992 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Taro | ECONAZOLE NITRATE | econazole nitrate | CREAM;TOPICAL | 076005-001 | Nov 26, 2002 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | AMMONIUM LACTATE | ammonium lactate | CREAM;TOPICAL | 075883-001 | Apr 10, 2003 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Taro
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Taro | TOPICORT | desoximetasone | SPRAY;TOPICAL | 204141-001 | Apr 11, 2013 | 5,990,100 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-002 | Jan 17, 2008 | 6,102,254 | ⤷ Try a Trial |
| Taro | PLIAGLIS | lidocaine; tetracaine | CREAM;TOPICAL | 021717-001 | Jun 29, 2006 | 5,919,479 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 6,071,523 | ⤷ Try a Trial |
| Taro | FLO-PRED | prednisolone acetate | SUSPENSION;ORAL | 022067-001 | Jan 17, 2008 | 6,399,079 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TARO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Lotion | 0.5% | ➤ Subscribe | 2011-03-16 |
| ➤ Subscribe | Topical Spray | 0.25% | ➤ Subscribe | 2013-12-18 |
International Patents for Taro Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Brazil | PI0612686 | ⤷ Try a Trial |
| European Patent Office | 2630951 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006130510 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2007005988 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2007019184 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Taro Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0957929 | SPC/GB06/021 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PEGAPTANIB, PREFERABLY IN THE FORM OF ITS SODIUM SALT; REGISTERED: UK EU/1/05/325/001 20060201 |
| 2435024 | 132021000000095 | Italy | ⤷ Try a Trial | PRODUCT NAME: UNA COMBINAZIONE DI FORMOTEROLO (INCLUSI SUOI SALI, ESTERI, SOLVATI O ENANTIOMERI FARMACEUTICAMENTE ACCETTABILI), GLICOPIRROLATO (INCLUSI SUOI SALI, ESTERI, SOLVATI O ENANTIOMERI FARMACEUTICAMENTE ACCETTABILI) E BUDESONIDE (INCLUSI SUOI SALI, ESTERI, SOLVATI O ENANTIOMERI FARMACEUTICAMENTE ACCETTABILI)(TRIXEO AEROSPHERE); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1498, 20201210 |
| 1458369 | 08C0024 | France | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE - PEROXYDE DE BENZOLE; REGISTRATION NO/DATE IN FRANCE: NL 33724 DU 20080123; REGISTRATION NO/DATE AT EEC: 40440 DU 20071218 |
| 1305329 | SPC/GB08/026 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FLUTICASONE FUROATE AND SOLVATES THEREOF; REGISTERED: UK EU/1/07/434/001 20080116; UK EU/1/07/434/002 20080116; UK EU/1/07/434/003 20080116 |
| 0347066 | SPC/GB02/049 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ESCITALOPRAM OXALATE; REGISTERED: SE 17084/85/86/87 20011207; UK PL 13761/0008 20020610; UK PC 13761/0009 20020610; UK PL 13761/0010 20020610; UK PL 13761/0011 20020610; UK PL 13761/0012 20020610; UK PL 13761/0013 20020610; UK PL 13761/00014 20020610; UK PL 13761/0015 20020610 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.