Takeda Pharms Usa Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TAKEDA PHARMS USA, and when can generic versions of TAKEDA PHARMS USA drugs launch?
TAKEDA PHARMS USA has forty approved drugs.
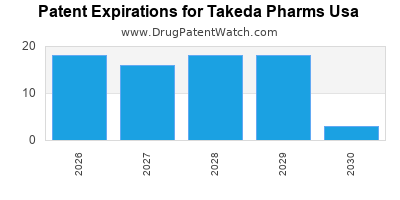
There are one hundred and six US patents protecting TAKEDA PHARMS USA drugs.
There are one thousand four hundred and twenty-nine patent family members on TAKEDA PHARMS USA drugs in fifty-nine countries and one hundred and eighty-eight supplementary protection certificates in nineteen countries.
Summary for Takeda Pharms Usa
| International Patents: | 1429 |
| US Patents: | 106 |
| Tradenames: | 43 |
| Ingredients: | 33 |
| NDAs: | 40 |
Drugs and US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-001 | Sep 30, 2013 | RX | Yes | No | 8,969,355*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Takeda Pharms Usa | TRINTELLIX | vortioxetine hydrobromide | TABLET;ORAL | 204447-003 | Sep 30, 2013 | DISCN | Yes | No | 9,125,910*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Takeda Pharms Usa | OMONTYS PRESERVATIVE FREE | peginesatide acetate | SOLUTION;INTRAVENOUS, SUBCUTANEOUS | 202799-001 | Mar 27, 2012 | DISCN | No | No | 7,084,245 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | NINLARO | ixazomib citrate | CAPSULE;ORAL | 208462-003 | Nov 20, 2015 | RX | Yes | Yes | 8,003,819 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Takeda Pharms Usa | CARBATROL | carbamazepine | CAPSULE, EXTENDED RELEASE;ORAL | 020712-001 | Sep 30, 1997 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Takeda Pharms Usa
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | INTUNIV | guanfacine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022037-004 | Sep 2, 2009 | 6,287,599*PED | ⤷ Try a Trial |
| Takeda Pharms Usa | OSENI | alogliptin benzoate; pioglitazone hydrochloride | TABLET;ORAL | 022426-005 | Jan 25, 2013 | 6,172,090 | ⤷ Try a Trial |
| Takeda Pharms Usa | ACTOS | pioglitazone hydrochloride | TABLET;ORAL | 021073-003 | Jul 15, 1999 | 6,329,404 | ⤷ Try a Trial |
| Takeda Pharms Usa | CARBATROL | carbamazepine | CAPSULE, EXTENDED RELEASE;ORAL | 020712-002 | Sep 30, 1997 | 6,221,392 | ⤷ Try a Trial |
| Takeda Pharms Usa | OSENI | alogliptin benzoate; pioglitazone hydrochloride | TABLET;ORAL | 022426-006 | Jan 25, 2013 | 7,078,381 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TAKEDA PHARMS USA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 1.2 g | ➤ Subscribe | 2009-12-16 |
| ➤ Subscribe | Extended-release Tablets | 1 mg, 2 mg, 3 mg and 4 mg | ➤ Subscribe | 2009-12-29 |
| ➤ Subscribe | Tablets | 0.6 mg | ➤ Subscribe | 2011-12-23 |
| ➤ Subscribe | Delayed-release Orally Disinte | 15 mg and 30 mg | ➤ Subscribe | 2006-12-27 |
| ➤ Subscribe | Tablets | 12.5 mg/500 mg and 12.5 mg/1000 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Delayed-release Capsule | 30 mg | ➤ Subscribe | 2010-11-30 |
| ➤ Subscribe | Tablets | 8 mg | ➤ Subscribe | 2009-07-22 |
| ➤ Subscribe | Extended-release Capsules | 37.5 mg and50 mg | ➤ Subscribe | 2017-08-03 |
| ➤ Subscribe | Tablets | 40 mg and 80 mg | ➤ Subscribe | 2013-02-13 |
| ➤ Subscribe | Capsules | 20 mg, 30 mg, 40 mg, 50 mg, 60 mg and 70 mg | ➤ Subscribe | 2011-02-23 |
| ➤ Subscribe | Injection | 10 mg/mL | ➤ Subscribe | 2015-08-25 |
| ➤ Subscribe | Oral Powder | 750 mg and 1000 mg | ➤ Subscribe | 2015-11-25 |
| ➤ Subscribe | Extended-release Tablets | 15 mg/1000 mg and 30 mg/1000 mg | ➤ Subscribe | 2011-09-23 |
| ➤ Subscribe | Tablets | 15 mg/500 mg and 15 mg/850 mg | ➤ Subscribe | 2008-03-06 |
| ➤ Subscribe | Delayed-release Pellets/Capsul | 15 mg and 30 mg | ➤ Subscribe | 2005-12-05 |
| ➤ Subscribe | Tablets | 30 mg/2 mg and 30 mg/4 mg | ➤ Subscribe | 2009-12-22 |
| ➤ Subscribe | Capsule | 60 mg | ➤ Subscribe | 2010-08-25 |
| ➤ Subscribe | Tablets | 6.25 mg, 12.5 mg and 25 mg | ➤ Subscribe | 2017-01-25 |
| ➤ Subscribe | Extended-release Capsules | 100 mg and 200 mg | ➤ Subscribe | 2006-02-02 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 15 mgand 20 mg | ➤ Subscribe | 2017-10-02 |
| ➤ Subscribe | Extended-release Capsules | 12.5 mg and 25 mg | ➤ Subscribe | 2017-08-07 |
| ➤ Subscribe | For Injection | 3.5 mg/vial | ➤ Subscribe | 2008-11-20 |
| ➤ Subscribe | Chewable Tablet | 500 mg, 750 mg and 1000 mg | ➤ Subscribe | 2008-10-27 |
International Patents for Takeda Pharms Usa Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 102105150 | ⤷ Try a Trial |
| Denmark | 1459737 | ⤷ Try a Trial |
| Denmark | 2133084 | ⤷ Try a Trial |
| Tunisia | 2010000411 | ⤷ Try a Trial |
| Canada | 2634923 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Takeda Pharms Usa Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2178888 | 17C1011 | France | ⤷ Try a Trial | PRODUCT NAME: IXAZOMIB ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES ET SES ESTERS TELS QUE LE CITRATE D'IXAZOMIB; REGISTRATION NO/DATE: EU/1/16/1094 20161123 |
| 1506211 | PA2014026,C1506211 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZINUM + METFORMINUM; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| 1412357 | C300357 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVA ARDBAAR ZOUT, IN HET BIJZONDER HET MONOFOSFAAT, EN METFORMINE DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER HET HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/08/455/001-014 20080716 |
| 2178888 | 1790016-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: IXAZOMIB AND PHARMACEUTICALLY ACCEPTABLE SALTS AND ESTERS THEREOF, SUCH AS IXAZOMIB CITRATE; REG. NO/DATE: EU/1/16/1094 20161123 |
| 1412357 | PA2008013,C1412357 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINUM PHOSPHAS MONOHYDRICUS, METFORMINI HYDROCHLORIDUM; REGISTRATION NO/DATE: EU/1/08/455/001 - EU/1/08/455/014 20080716 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.