Am Regent Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AM REGENT, and what generic alternatives to AM REGENT drugs are available?
AM REGENT has seventy-eight approved drugs.
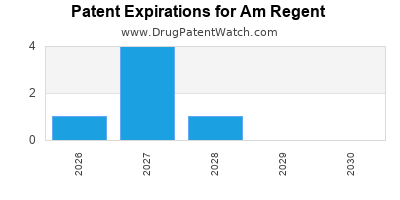
There are seven US patents protecting AM REGENT drugs.
There are sixty-seven patent family members on AM REGENT drugs in thirty-two countries and one hundred and forty-three supplementary protection certificates in fifteen countries.
Drugs and US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am Regent | NICARDIPINE HYDROCHLORIDE | nicardipine hydrochloride | INJECTABLE;INJECTION | 090534-001 | Nov 17, 2009 | AP | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | SELENIOUS ACID | selenious acid | SOLUTION;INTRAVENOUS | 209379-001 | Apr 30, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | ZINC SULFATE | zinc sulfate | SOLUTION;INTRAVENOUS | 209377-002 | Jul 18, 2019 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | EPINEPHRINE | epinephrine | SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS | 207568-001 | Jul 6, 2018 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | FOSPHENYTOIN SODIUM | fosphenytoin sodium | INJECTABLE;INJECTION | 090099-001 | May 13, 2010 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | VASOPRESSIN | vasopressin | SOLUTION;INTRAVENOUS | 212593-002 | Jun 9, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-004 | Feb 4, 2022 | 11,123,321 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-001 | Jul 25, 2013 | 11,291,645 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,291,645 | ⤷ Try a Trial |
| Am Regent | DEXFERRUM | ferric oxyhydroxide | INJECTABLE;INJECTION | 040024-001 | Feb 23, 1996 | 5,624,668 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 11,123,321 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-001 | Jul 25, 2013 | 11,123,321 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Am Regent Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Peru | 20040571 | ⤷ Try a Trial |
| Russian Federation | 2005115455 | ⤷ Try a Trial |
| New Zealand | 539243 | ⤷ Try a Trial |
| Norway | 335770 | ⤷ Try a Trial |
| South Korea | 20140117678 | ⤷ Try a Trial |
| Norway | 333008 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Am Regent Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1853250 | PA2014022 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PACLITAXELUM; REGISTRATION NO/DATE: EU/1/07/428/001-002 20131220 |
| 2782584 | LUC00245 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), EVENTUELLEMENT SOUS FORME D'UN SEL, HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (Y COMPRIS SOUS FORME HEMIHYDRATEE), ET DE LA PROGESTERONE; AUTHORISATION NUMBER AND DATE: BE582231 20210701 |
| 1718641 | SPC/GB12/028 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: AZILSARTAN MEDOXOMIL AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING THE POTASSIUM SALT; REGISTERED: UK EU/1/11/734/001-011 20111209 |
| 0398460 | C300221 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON EN ETHINYLESTRADIOL; REGISTRATION NO/DATE: RVG 23827 20000307 |
| 2957286 | SPC/GB19/003 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PATIROMER SORBITEX CALCIUM; REGISTERED: UK EU/1/17/1179/001(NI) 20170721; UK EU/1/17/1179/002(NI) 20170721; UK EU/1/17/1179/003(NI) 20170721; UK EU/1/17/1179/004(NI) 20170721; UK EU/1/17/1179/005(NI) 20170721; UK EU/1/17/1179/006(NI) 20170721; UK EU/1/17/1179/007(NI) 20170721; UK EU/1/17/1179/008(NI) 20170721; UK EU/1/17/1179/009(NI) 20170721; UK PLGB 50784/0002-0001 20170721; UK PLGB 50784/0003-0001 20170721; UK PLGB 50784/0004-0001 20170721 |
| 2782584 | 122021000080 | Germany | ⤷ Try a Trial | PRODUCT NAME: ZUSAMMENSETZUNG, DIE SOWOHL ESTRADIOL (17SS-ESTRADIOL), GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, HYDRATS ODER SOLVATS DAVON (EINSCHLIESSLICH IN FORM EINES HEMIHYDRATS), ALS AUCH PROGESTERON ENTHAELT; NAT. REGISTRATION NO/DATE: 2205034.00.00 20210924; FIRST REGISTRATION: BELGIEN BE582231 20210406 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.