ISTURISA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Isturisa, and when can generic versions of Isturisa launch?
Isturisa is a drug marketed by Recordati Rare and is included in one NDA. There are six patents protecting this drug.
This drug has one hundred and thirty-five patent family members in forty-three countries.
The generic ingredient in ISTURISA is osilodrostat phosphate. One supplier is listed for this compound. Additional details are available on the osilodrostat phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Isturisa
Isturisa was eligible for patent challenges on March 6, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 6, 2035. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ISTURISA
| International Patents: | 135 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 50 |
| Patent Applications: | 54 |
| Drug Prices: | Drug price information for ISTURISA |
| What excipients (inactive ingredients) are in ISTURISA? | ISTURISA excipients list |
| DailyMed Link: | ISTURISA at DailyMed |
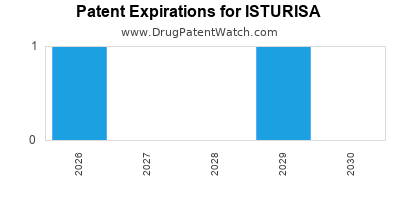

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ISTURISA
Generic Entry Date for ISTURISA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for ISTURISA
Anatomical Therapeutic Chemical (ATC) Classes for ISTURISA
US Patents and Regulatory Information for ISTURISA
ISTURISA is protected by six US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ISTURISA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ISTURISA
Pharmaceutical dosage forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical dosage forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CUSHING'S DISEASE
Organic compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of an adrenal hormone-modifying agent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CUSHING'S DISEASE
Organic compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of an adrenal hormone-modifying agent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting ISTURISA
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE TREATMENT OF ADULT PATIENTS WITH CUSHING’S DISEASE FOR WHOM PITUITARY SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
Exclusivity Expiration: ⤷ Try a Trial
International Patents for ISTURISA
When does loss-of-exclusivity occur for ISTURISA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1116
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 15287336
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2016030243
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 54393
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 17000026
Estimated Expiration: ⤷ Try a Trial
China
Patent: 6470704
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0181406
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 20749
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 66596
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 17008187
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 3685
Estimated Expiration: ⤷ Try a Trial
Patent: 1790140
Estimated Expiration: ⤷ Try a Trial
Patent: 1991359
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 66596
Estimated Expiration: ⤷ Try a Trial
Patent: 12278
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 39037
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9374
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 31136
Estimated Expiration: ⤷ Try a Trial
Patent: 17520590
Estimated Expiration: ⤷ Try a Trial
Patent: 19194221
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 66596
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 16017315
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 170201
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 016502540
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 66596
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 66596
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201610227T
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 66596
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2468610
Estimated Expiration: ⤷ Try a Trial
Patent: 170029491
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 86704
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 07682
Estimated Expiration: ⤷ Try a Trial
Patent: 1613586
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 16000557
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ISTURISA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | I487708 | ⤷ Try a Trial | |
| Ecuador | SP088201 | DERIVADOS DE IMIDAZOLO CONDENSADO PARA LA INHIBICIÓN DE SINTASA DE ALDOSTERONA Y AROMATASA | ⤷ Try a Trial |
| Cyprus | 1120749 | ⤷ Try a Trial | |
| European Patent Office | 1919916 | DERIVES IMIDAZOLO CONDENSES UTILISES POUR INHIBER L'ALDOSTERONE SYNTHASE ET L'AROMATASE (CONDENSED IMIDAZOLO DERIVATIVES FOR THE INHIBITION OF ALDOSTERONE SYNTHASE AND AROMATASE) | ⤷ Try a Trial |
| Mexico | 2016017315 | FORMAS DE DOSIFICACION FARMACEUTICA. (PHARMACEUTICAL DOSAGE FORMS.) | ⤷ Try a Trial |
| Eurasian Patent Organization | 200800488 | КОНДЕНСИРОВАННЫЕ ПРОИЗВОДНЫЕ ИМИДАЗОЛА ДЛЯ ИНГИБИРОВАНИЯ АЛЬДОСТЕРОНСИНТАЗЫ И АРОМАТАЗЫ | ⤷ Try a Trial |
| Poland | 2523731 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ISTURISA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2523731 | 132020000000052 | Italy | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT(ISTURISA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/19/1407, 20200113 |
| 2523731 | C20200013 00309 | Estonia | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAAT;REG NO/DATE: EU/1/19/1407 13.01.2020 |
| 2523731 | 2020C/520 | Belgium | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT HIERVAN, INCLUSIEF OSILODROSTAT DIWATERSTOFFOSFAAT; AUTHORISATION NUMBER AND DATE: EU/1/19/1407 20200113 |
| 2523731 | CR 2020 00025 | Denmark | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, HERUNDER OSILODROSTATDIHYDROGENFOSFAT; REG. NO/DATE: EU/1/19/1407 20200113 |
| 2523731 | 301043 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, MET INBEGRIP VAN OSILODROSTATDIWATERSTOFFOSFAAT; REGISTRATION NO/DATE: EU/1/19/1407 20200113 |
| 2523731 | SPC/GB20/034 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT, OR OSILODROSTAT PHOSPHATE; REGISTERED: UK EU/1/19/1407/001(NI) 20200113; UK EU/1/19/1407/002(NI) 20200113; UK EU/1/19/1407/003(NI) 20200113; UK PLGB 15266/0029-0001 20200113; UK PLGB 15266/0030-0001 20200113; UK PLGB 15266/0031-0001 20200113 |
| 2523731 | 2020012 | Norway | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT ELLER ET FARMASOEYTISK AKSEPTABELT SALT DERAV, INKLUDERT OSILODROSTAT DIHYDROGENFOSFAT; REG. NO/DATE: EU/1/19/1407 20200203 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


