FOSINOPRIL SODIUM Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Fosinopril Sodium, and what generic alternatives are available?
Fosinopril Sodium is a drug marketed by Actavis Labs Fl Inc, Apotex Inc, Aurobindo Pharma Ltd, Chartwell Rx, Invagen Pharms, Prinston Inc, Ranbaxy Labs Ltd, Teva, Upsher Smith Labs, Watson Labs, Ani Pharms, Aurobindo Pharma, Avet Lifesciences, Mylan, Sandoz, and Sun Pharm Inds Ltd. and is included in twenty NDAs.
The generic ingredient in FOSINOPRIL SODIUM is fosinopril sodium; hydrochlorothiazide. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the fosinopril sodium; hydrochlorothiazide profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Fosinopril Sodium
A generic version of FOSINOPRIL SODIUM was approved as fosinopril sodium; hydrochlorothiazide by SANDOZ on September 28th, 2005.
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for FOSINOPRIL SODIUM?
- What are the global sales for FOSINOPRIL SODIUM?
- What is Average Wholesale Price for FOSINOPRIL SODIUM?
Summary for FOSINOPRIL SODIUM
| US Patents: | 0 |
| Applicants: | 16 |
| NDAs: | 20 |
| Finished Product Suppliers / Packagers: | 7 |
| Raw Ingredient (Bulk) Api Vendors: | 111 |
| Clinical Trials: | 5 |
| Patent Applications: | 1,800 |
| Drug Prices: | Drug price information for FOSINOPRIL SODIUM |
| DailyMed Link: | FOSINOPRIL SODIUM at DailyMed |
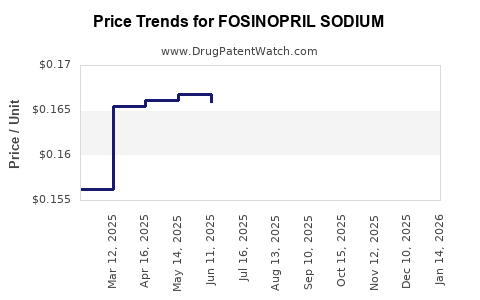
See drug prices for FOSINOPRIL SODIUM
Recent Clinical Trials for FOSINOPRIL SODIUM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| First Affiliated Hospital of Zhejiang University | Phase 4 |
| The Second Affiliated Hospital of Dalian Medical University | Phase 4 |
| Sichuan Provincial People's Hospital | Phase 4 |
Pharmacology for FOSINOPRIL SODIUM
| Drug Class | Angiotensin Converting Enzyme Inhibitor |
| Mechanism of Action | Angiotensin-converting Enzyme Inhibitors |


