FIDAXOMICIN Drug Patent Profile
✉ Email this page to a colleague
When do Fidaxomicin patents expire, and what generic alternatives are available?
Fidaxomicin is a drug marketed by Actavis Labs Fl and is included in one NDA.
The generic ingredient in FIDAXOMICIN is fidaxomicin. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the fidaxomicin profile page.
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for FIDAXOMICIN?
- What are the global sales for FIDAXOMICIN?
- What is Average Wholesale Price for FIDAXOMICIN?
Summary for FIDAXOMICIN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 61 |
| Clinical Trials: | 36 |
| Patent Applications: | 552 |
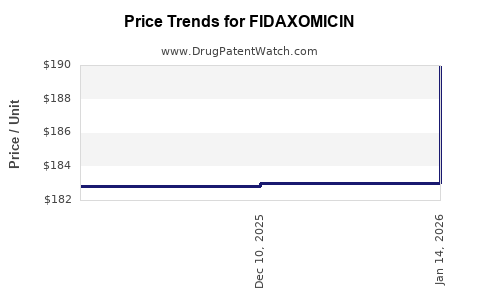
| Drug Prices: | Drug price information for FIDAXOMICIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for FIDAXOMICIN |
| DailyMed Link: | FIDAXOMICIN at DailyMed |
Recent Clinical Trials for FIDAXOMICIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hospital Universitario Evangelico de Curitiba | Phase 3 |
| Hellenic Institute for the Study of Sepsis | Phase 2 |
| Benoit Guery | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for FIDAXOMICIN
Paragraph IV (Patent) Challenges for FIDAXOMICIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| DIFICID | Tablets | fidaxomicin | 200 mg | 201699 | 1 | 2015-05-27 |
US Patents and Regulatory Information for FIDAXOMICIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actavis Labs Fl | FIDAXOMICIN | fidaxomicin | TABLET;ORAL | 208443-001 | Jan 16, 2024 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for FIDAXOMICIN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Tillotts Pharma GmbH | Dificlir | fidaxomicin | EMEA/H/C/002087 Dificlir film-coated tablets is indicated for the treatment of Clostridioides difficile infections (CDI) also known as C. difficile-associated diarrhoea (CDAD) in adult and paediatric patients with a body weight of at least 12.5 kg.Consideration should be given to official guidelines on the appropriate use of antibacterial agents.Dificlir granules for oral suspension is indicated for the treatment of Clostridioides difficile infections (CDI) also known as C. difficile-associated diarrhoea (CDAD) in adults and paediatric patients from birth to < 18 years of age.Consideration should be given to official guidelines on the appropriate use of antibacterial agents. |
Authorised | no | no | no | 2011-12-05 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


