DIAZOXIDE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Diazoxide, and what generic alternatives are available?
Diazoxide is a drug marketed by Abraxis Pharm, E5 Pharma Inc, and Novitium Pharma. and is included in three NDAs.
The generic ingredient in DIAZOXIDE is diazoxide. There are five drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the diazoxide profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Diazoxide
A generic version of DIAZOXIDE was approved as diazoxide by E5 PHARMA INC on December 20th, 2019.
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for DIAZOXIDE?
- What are the global sales for DIAZOXIDE?
- What is Average Wholesale Price for DIAZOXIDE?
Summary for DIAZOXIDE
| US Patents: | 0 |
| Applicants: | 3 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 112 |
| Clinical Trials: | 41 |
| Patent Applications: | 3,824 |
| Drug Prices: | Drug price information for DIAZOXIDE |
| DailyMed Link: | DIAZOXIDE at DailyMed |
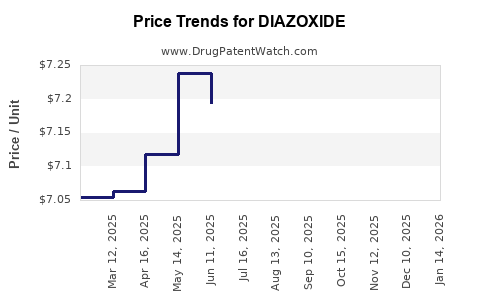
Recent Clinical Trials for DIAZOXIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Johns Hopkins University | Phase 1 |
| Columbia University | Phase 1 |
| Soleno Therapeutics, Inc. | Phase 2 |
Medical Subject Heading (MeSH) Categories for DIAZOXIDE
Anatomical Therapeutic Chemical (ATC) Classes for DIAZOXIDE
US Patents and Regulatory Information for DIAZOXIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abraxis Pharm | DIAZOXIDE | diazoxide | INJECTABLE;INJECTION | 071519-001 | Aug 26, 1987 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| E5 Pharma Inc | DIAZOXIDE | diazoxide | SUSPENSION;ORAL | 211050-001 | Dec 20, 2019 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Novitium Pharma | DIAZOXIDE | diazoxide | SUSPENSION;ORAL | 210799-001 | Jul 8, 2020 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


