AMONDYS 45 Drug Patent Profile
✉ Email this page to a colleague
When do Amondys 45 patents expire, and what generic alternatives are available?
Amondys 45 is a drug marketed by Sarepta Theraps Inc and is included in one NDA. There are six patents protecting this drug.
This drug has eighty-seven patent family members in twenty-four countries.
The generic ingredient in AMONDYS 45 is casimersen. One supplier is listed for this compound. Additional details are available on the casimersen profile page.
DrugPatentWatch® Generic Entry Outlook for Amondys 45
Amondys 45 will be eligible for patent challenges on February 25, 2025. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 12, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for AMONDYS 45
| International Patents: | 87 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| What excipients (inactive ingredients) are in AMONDYS 45? | AMONDYS 45 excipients list |
| DailyMed Link: | AMONDYS 45 at DailyMed |
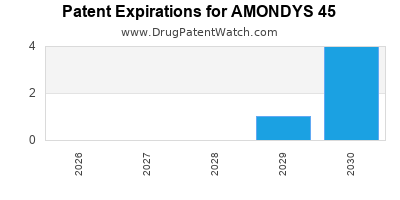
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AMONDYS 45
Generic Entry Date for AMONDYS 45*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for AMONDYS 45
| Drug Class | Antisense Oligonucleotide |
| Physiological Effect | Increased Protein Synthesis |
Anatomical Therapeutic Chemical (ATC) Classes for AMONDYS 45
US Patents and Regulatory Information for AMONDYS 45
AMONDYS 45 is protected by eight US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AMONDYS 45 is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting AMONDYS 45
Antisense molecules and methods for treating pathologies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Antisense molecules and methods for treating pathologies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) IN PATIENTS WHO HAVE A MUTATION OF THE DMD GENE THAT IS AMENABLE TO EXON 45 SKIPPING BY RESTORING AN MRNA READING FRAME TO INDUCE DYSTROPHIN PROTEIN PRODUCTION
Antisense molecules and methods for treating pathologies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Antisense molecules and methods for treating pathologies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) IN PATIENTS WHO HAVE A MUTATION OF THE DMD GENE THAT IS AMENABLE TO EXON 45 SKIPPING
Antisense molecules and methods for treating pathologies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) IN PATIENTS WHO HAVE A MUTATION OF THE DMD GENE THAT IS AMENABLE TO EXON 45 SKIPPING BY RESTORING AN MRNA READING FRAME TO INDUCE DYSTROPHIN PROTEIN PRODUCTION
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) IN PATIENTS WHO HAVE A MUTATION OF THE DMD GENE THAT IS AMENABLE TO EXON 45 SKIPPING BY INDUCING EXON-SKIPPING OF EXON 45
Antisense oligonucleotides for inducing exon skipping and methods of use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) IN PATIENTS WHO HAVE A MUTATION OF THE DMD GENE THAT IS AMENABLE TO EXON 45 SKIPPING
FDA Regulatory Exclusivity protecting AMONDYS 45
TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) IN PATIENTS WHO HAVE A CONFIRMED MUTATION OF THE DMD GENE THAT IS AMENABLE TO EXON 45 SKIPPING
Exclusivity Expiration: ⤷ Try a Trial
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarepta Theraps Inc | AMONDYS 45 | casimersen | SOLUTION;INTRAVENOUS | 213026-001 | Feb 25, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sarepta Theraps Inc | AMONDYS 45 | casimersen | SOLUTION;INTRAVENOUS | 213026-001 | Feb 25, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Sarepta Theraps Inc | AMONDYS 45 | casimersen | SOLUTION;INTRAVENOUS | 213026-001 | Feb 25, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AMONDYS 45
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sarepta Theraps Inc | AMONDYS 45 | casimersen | SOLUTION;INTRAVENOUS | 213026-001 | Feb 25, 2021 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sarepta Theraps Inc | AMONDYS 45 | casimersen | SOLUTION;INTRAVENOUS | 213026-001 | Feb 25, 2021 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AMONDYS 45
When does loss-of-exclusivity occur for AMONDYS 45?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10317599
Estimated Expiration: ⤷ Try a Trial
Patent: 16202924
Estimated Expiration: ⤷ Try a Trial
Patent: 18202105
Estimated Expiration: ⤷ Try a Trial
Patent: 20260498
Estimated Expiration: ⤷ Try a Trial
Patent: 23203103
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2012011195
Estimated Expiration: ⤷ Try a Trial
Patent: 2023023194
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 80563
Estimated Expiration: ⤷ Try a Trial
China
Patent: 3003430
Estimated Expiration: ⤷ Try a Trial
Patent: 5838714
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0181824
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 21198
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 99249
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 99249
Estimated Expiration: ⤷ Try a Trial
Patent: 31603
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 40445
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9753
Estimated Expiration: ⤷ Try a Trial
Patent: 5707
Estimated Expiration: ⤷ Try a Trial
Patent: 4525
Estimated Expiration: ⤷ Try a Trial
Patent: 6947
Estimated Expiration: ⤷ Try a Trial
Patent: 7299
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 63678
Estimated Expiration: ⤷ Try a Trial
Patent: 94393
Estimated Expiration: ⤷ Try a Trial
Patent: 25449
Estimated Expiration: ⤷ Try a Trial
Patent: 36830
Estimated Expiration: ⤷ Try a Trial
Patent: 13510561
Estimated Expiration: ⤷ Try a Trial
Patent: 16198105
Estimated Expiration: ⤷ Try a Trial
Patent: 18082714
Estimated Expiration: ⤷ Try a Trial
Patent: 19141073
Estimated Expiration: ⤷ Try a Trial
Patent: 22001053
Estimated Expiration: ⤷ Try a Trial
Patent: 24029262
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 99249
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 6359
Estimated Expiration: ⤷ Try a Trial
Patent: 6534
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 99249
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 99249
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 079
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 99249
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1958491
Estimated Expiration: ⤷ Try a Trial
Patent: 2000762
Estimated Expiration: ⤷ Try a Trial
Patent: 2113306
Estimated Expiration: ⤷ Try a Trial
Patent: 2239374
Estimated Expiration: ⤷ Try a Trial
Patent: 2366851
Estimated Expiration: ⤷ Try a Trial
Patent: 2487132
Estimated Expiration: ⤷ Try a Trial
Patent: 2581868
Estimated Expiration: ⤷ Try a Trial
Patent: 130084595
Estimated Expiration: ⤷ Try a Trial
Patent: 180004745
Estimated Expiration: ⤷ Try a Trial
Patent: 190084360
Estimated Expiration: ⤷ Try a Trial
Patent: 200055161
Estimated Expiration: ⤷ Try a Trial
Patent: 210041130
Estimated Expiration: ⤷ Try a Trial
Patent: 220031125
Estimated Expiration: ⤷ Try a Trial
Patent: 230010814
Estimated Expiration: ⤷ Try a Trial
Patent: 230137491
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 93459
Estimated Expiration: ⤷ Try a Trial
Turkey
Patent: 1816523
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AMONDYS 45 around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 5752601 | ⤷ Try a Trial | |
| Japan | 6525449 | ⤷ Try a Trial | |
| Denmark | 2206781 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


