AKEEGA Drug Patent Profile
✉ Email this page to a colleague
When do Akeega patents expire, and what generic alternatives are available?
Akeega is a drug marketed by Janssen Biotech and is included in one NDA. There are eight patents protecting this drug.
This drug has two hundred and eighty-five patent family members in fifty-five countries.
The generic ingredient in AKEEGA is abiraterone acetate; niraparib tosylate. There are twenty-five drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the abiraterone acetate; niraparib tosylate profile page.
DrugPatentWatch® Generic Entry Outlook for Akeega
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 27, 2038. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for AKEEGA
| International Patents: | 285 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for AKEEGA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AKEEGA |
| What excipients (inactive ingredients) are in AKEEGA? | AKEEGA excipients list |
| DailyMed Link: | AKEEGA at DailyMed |
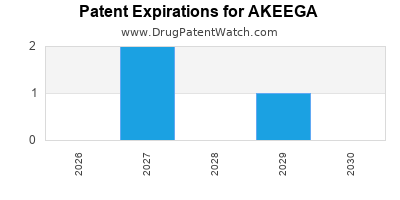

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AKEEGA
Generic Entry Date for AKEEGA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for AKEEGA
Anatomical Therapeutic Chemical (ATC) Classes for AKEEGA
US Patents and Regulatory Information for AKEEGA
AKEEGA is protected by eight US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AKEEGA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting AKEEGA
Niraparib compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of treating prostate cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR TREATING METASTATIC CASTRATION-RESISTANT PROSTATE CANCER (MCRPC), WHEREIN THE CANCER IS ASSOCIATED WITH A DELETERIOUS BRCA-MUTATION
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
DNA damage repair inhibitors for the treatment of cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR TREATING METASTATIC CASTRATION-RESISTANT PROSTATE CANCER (MCRPC), WHEREIN THE CANCER IS ASSOCIATED WITH A DELETERIOUS BRCA-MUTATION
Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
DNA damage repair inhibitors for treatment of cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR TREATING METASTATIC CASTRATION-RESISTANT PROSTATE CANCER (MCRPC), WHEREIN THE CANCER IS ASSOCIATED WITH A DELETERIOUS BRCA-MUTATION
Pharmaceutically acceptable salts of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of RNAI inhibiting PARP activity for the manufacture of a medicament for the treatment of cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR TREATING METASTATIC CASTRATION-RESISTANT PROSTATE CANCER (MCRPC), WHEREIN THE CANCER IS ASSOCIATED WITH A DELETERIOUS BRCA-MUTATION
FDA Regulatory Exclusivity protecting AKEEGA
NEW PRODUCT
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-001 | Aug 11, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-002 | Aug 11, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-001 | Aug 11, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-001 | Aug 11, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-002 | Aug 11, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-001 | Aug 11, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for AKEEGA
When does loss-of-exclusivity occur for AKEEGA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 18246214
Estimated Expiration: ⤷ Try a Trial
Patent: 21245223
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2019020211
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 58375
Estimated Expiration: ⤷ Try a Trial
China
Patent: 0944638
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1992177
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 00314
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9630
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 20512350
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 19011496
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201909011P
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 200014736
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 61476
Estimated Expiration: ⤷ Try a Trial
Patent: 1840315
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AKEEGA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2009532452 | ⤷ Try a Trial | |
| Mexico | 2019011496 | COMPOSICIONES DE NIRAPARIB. (NIRAPARIB COMPOSITIONS.) | ⤷ Try a Trial |
| Poland | 2336120 | ⤷ Try a Trial | |
| Mexico | 2019001224 | METODOS PARA TRATAR EL CANCER DE PROSTATA. (METHODS OF TREATING PROSTATE CANCER.) | ⤷ Try a Trial |
| Montenegro | 03481 | INDAZOLI SUPSTITUISANI AMIDOM KAO INHIBITORI POLI(ADP-RIBOZA)POLIMERAZE (PARP) (AMIDE SUBSTITUTED INDAZOLES AS POLY(ADP-RIBOSE)POLYMERASE (PARP) INHIBITORS) | ⤷ Try a Trial |
| Austria | 516353 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AKEEGA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1633724 | 213 50005-2015 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: OLAPARIB; REGISTRATION NO/DATE: EU/1/14/959 20141218 |
| 2109608 | 18C1019 | France | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB OU L'UN DE SES SELS,STEREOISOMERES OU TAUTOMERES PHARMACEUTIQUEMENT ACCEPTABLES EN PARTICULIER LE TOSYLATE OU HYDRATE DE TOSYLATE,PLUS PARTICULIEREMENT LE TOSYLATE DE NIRAPARIB MONOHYDRATE; REGISTRATION NO/DATE: EU/1/17/1235 20171120 |
| 2109608 | 667 | Finland | ⤷ Try a Trial | |
| 2109608 | LUC00072 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE, STEREOISOMERE OU TAUTOMERE DE CELUI-CI, NOTAMMENT LE TOSYLATE OU UN HYDRATE, PARTICULIEREMENT LE TOSYLATE MONOHYDRATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1235 20171120 |
| 0633893 | 2012/003 | Ireland | ⤷ Try a Trial | PRODUCT NAME: ZYTIGA (ABIRATERONE) "ABIRATERONE AND ACID ADDITION SALTS AND 3-ESTERS THEREOF, ESPECIALLY ABIRATERONE ACETATE"; REGISTRATION NO/DATE: EU/1/11/714/001 20110905 |
| 2109608 | 300937 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB, OF EEN TAUTOMEER DAARVAN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER HET TOSYLAAT OF EEN HYDRAAT, MEER IN HET BIJZONDER HET TOSYLAAT MONOHYDRAAT; REGISTRATION NO/DATE: EU/1/17/1235 20171120 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


