SALIX Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SALIX, and when can generic versions of SALIX drugs launch?
SALIX has eighteen approved drugs.
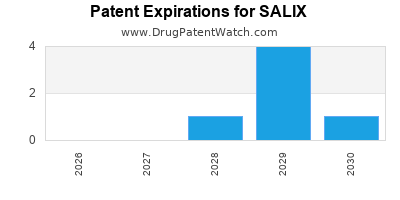
There are seventy-five US patents protecting SALIX drugs.
There are six hundred and forty-nine patent family members on SALIX drugs in fifty-five countries and ninety-seven supplementary protection certificates in twelve countries.
Summary for SALIX
| International Patents: | 649 |
| US Patents: | 75 |
| Tradenames: | 15 |
| Ingredients: | 13 |
| NDAs: | 18 |
| Patent Litigation for SALIX: | See patent lawsuits for SALIX |
| PTAB Cases with SALIX as patent owner: | See PTAB cases with SALIX as patent owner |
Drugs and US Patents for SALIX
Expired US Patents for SALIX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Salix | ZEGERID | omeprazole; sodium bicarbonate | CAPSULE;ORAL | 021849-002 | Feb 27, 2006 | 6,645,988 | ⤷ Try a Trial |
| Salix | APRISO | mesalamine | CAPSULE, EXTENDED RELEASE;ORAL | 022301-001 | Oct 31, 2008 | 8,911,778 | ⤷ Try a Trial |
| Salix | ZEGERID | omeprazole; sodium bicarbonate | FOR SUSPENSION;ORAL | 021636-002 | Dec 21, 2004 | RE45198 | ⤷ Try a Trial |
| Salix | UCERIS | budesonide | TABLET, EXTENDED RELEASE;ORAL | 203634-001 | Jan 14, 2013 | 9,532,954 | ⤷ Try a Trial |
| Salix | ZEGERID | omeprazole; sodium bicarbonate | FOR SUSPENSION;ORAL | 021636-002 | Dec 21, 2004 | 7,399,772 | ⤷ Try a Trial |
| Salix | ZEGERID | omeprazole; sodium bicarbonate | CAPSULE;ORAL | 021849-002 | Feb 27, 2006 | 7,399,772 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SALIX drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Orally Disintegrating Tablets | 5 mg and 10 mg | ➤ Subscribe | 2010-08-24 |
| ➤ Subscribe | Tablets | 550 mg | ➤ Subscribe | 2015-12-18 |
| ➤ Subscribe | Extended-release Tablets | 9 mg | ➤ Subscribe | 2013-03-11 |
| ➤ Subscribe | Capsules | 20 mg/1100 mg and 40 mg/1100 mg | ➤ Subscribe | 2007-04-30 |
| ➤ Subscribe | Powder for Oral Suspension | 20mg/1680mg per packet | ➤ Subscribe | 2007-11-13 |
| ➤ Subscribe | Tablets | 40 mg and 120 mg | ➤ Subscribe | 2010-03-17 |
| ➤ Subscribe | Injection | 8 mg/0.4 mL | ➤ Subscribe | 2015-09-08 |
| ➤ Subscribe | For Oral Solution | 100 g, 7.5 g, 2.691 g, 1.015 g, 5.9 g and 4.7 g per pouch | ➤ Subscribe | 2007-11-27 |
| ➤ Subscribe | Tablets | 1.102 g and 0.398 g | ➤ Subscribe | 2008-04-09 |
| ➤ Subscribe | Tablets | 200 mg | ➤ Subscribe | 2019-01-28 |
| ➤ Subscribe | Extended-release Capsules | 0.375 g | ➤ Subscribe | 2012-04-03 |
| ➤ Subscribe | Powder for Oral Suspension | 40 mg/1680 mg per packet | ➤ Subscribe | 2007-08-24 |
| ➤ Subscribe | For Oral Solution | 140 g, 5.2 g, 2.2.g, 48.11 g, 9g and 7.54 g per pouch | ➤ Subscribe | 2018-12-06 |
| ➤ Subscribe | Injection | 12 mg/0.6 mL | ➤ Subscribe | 2015-07-22 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2016-09-06 |
International Patents for SALIX Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Russian Federation | 2007136430 | ⤷ Try a Trial |
| Austria | 251449 | ⤷ Try a Trial |
| Japan | 2014507462 | ⤷ Try a Trial |
| United Kingdom | 0224909 | ⤷ Try a Trial |
| Russian Federation | 2600790 | ⤷ Try a Trial |
| Japan | 2003501457 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SALIX Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0480717 | SPC/GB98/025 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: MONTELUKAST, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, PREFERABLY MONTELUKAST SODIUM; REGISTERED: FI 12766 19970825; FI 12767 19970825; UK 00025/0357 19980115; UK 00025/0358 19980115 |
| 1175904 | 2007C/048 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ALENDRONATE DE SODIUM/COLECALCIFEROL; AUTHORISATION NUMBER AND DATE: EU/1/05/310/001 20050826 |
| 0565634 | 06C0030 | France | ⤷ Try a Trial | PRODUCT NAME: EPROSARTAN MESYLATE; HYDROCHLOROTHIAZIDE; NAT. REGISTRATION NO/DATE: NL 32075 20060623; FIRST REGISTRATION: LI - 55783 01 20020607 |
| 3141251 | 132021000000044 | Italy | ⤷ Try a Trial | PRODUCT NAME: UN PRODOTTO MEDICINALE COSTITUITO DA UNA COMBINAZIONE DI UNA PRIMA DOSE DI COMPOSIZIONE FARMACEUTICA E UNA SECONDA DOSE DI COMPOSIZIONE FARMACEUTICA, LA PRIMA DOSE DI COMPOSIZIONE FARMACEUTICA E COSTITUITA DAGLI INGREDIENTI ATTIVI POLIETILENGLICOLE, SOLFATO DI SODIO, CLORURO DI SODIO E CLORURO DI POTASSIO E LA SECONDA DOSE DI COMPOSIZIONE FARMACEUTICA E COSTITUITA DAGLI INGREDIENTI ATTIVI POLIETILENGLICOLE, ACIDO ASCORBICO, ASCORBATO DI SODIO, CLORURO DI SODIO E CLORURO DI POTASSIO.(PLENVU); AUTHORISATION NUMBER(S) AND DATE(S): IS/1/17/083/01, 20171016;045671017, 045671029, 045671031, 045671043, 045671056, 20171213 |
| 1507558 | 2012/018 | Ireland | ⤷ Try a Trial | PRODUCT NAME: ALISKIREN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AMLODIPINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; NAT REGISTRATION NO/DATE: EU/1/11/730/001-060 20111122; FIRST REGISTRATION NO/DATE: SWITZERLAND 6167801-6167805 20110705 |
| 3141251 | C202130017 | Spain | ⤷ Try a Trial | PRODUCT NAME: COMBINACION DE PRINCIPIOS ACTIVOS EN DOS DOSIS, EN LA QUE LA PRIMERA DOSIS CONSISTE EN LOS PRINCIPIOS ACTIVOS POLIETILENGLICOL, SULFATO DE SODIO, CLORURO DE SODIO Y CLORURO DE POTASIO, Y LA SEGUNDA DOSIS CONSISTE EN LOS PRINCIPIOS ACTIVOS POLIETILENGLICOL, ACIDO ASCORBICO, ASCORBATO DE SODIO, CLORURO DE SODIO Y CLORURO DE POTASIO.; NATIONAL AUTHORISATION NUMBER: 82959-SE/H/1801/001/DC; DATE OF AUTHORISATION: 20180525; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): IS/1/17/083/01; DATE OF FIRST AUTHORISATION IN EEA: 20171016 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.