OSMOPREP Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Osmoprep, and what generic alternatives are available?
Osmoprep is a drug marketed by Salix Pharms and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has twenty-nine patent family members in eleven countries.
The generic ingredient in OSMOPREP is sodium phosphate, dibasic, anhydrous; sodium phosphate, monobasic, monohydrate. There are one thousand four hundred and seventy-two drug master file entries for this compound. Additional details are available on the sodium phosphate, dibasic, anhydrous; sodium phosphate, monobasic, monohydrate profile page.
DrugPatentWatch® Generic Entry Outlook for Osmoprep
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for OSMOPREP
| International Patents: | 29 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Clinical Trials: | 1 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for OSMOPREP |
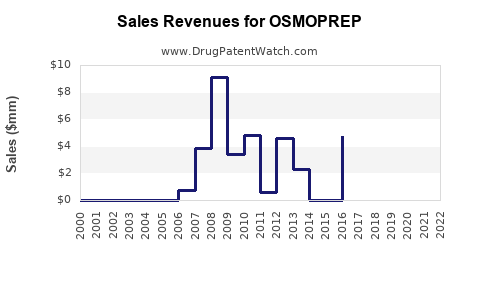
| Drug Sales Revenues: | Drug sales revenues for OSMOPREP |
| What excipients (inactive ingredients) are in OSMOPREP? | OSMOPREP excipients list |
| DailyMed Link: | OSMOPREP at DailyMed |

Recent Clinical Trials for OSMOPREP
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Bausch Health Americas, Inc. | Phase 4 |
| Valeant Pharmaceuticals International, Inc. | Phase 4 |
Anatomical Therapeutic Chemical (ATC) Classes for OSMOPREP
Paragraph IV (Patent) Challenges for OSMOPREP
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OSMOPREP | Tablets | sodium phosphate, dibasic, anhydrous; sodium phosphate, monobasic, monohydrate | 1.102 g and 0.398 g | 021892 | 1 | 2008-04-09 |
US Patents and Regulatory Information for OSMOPREP
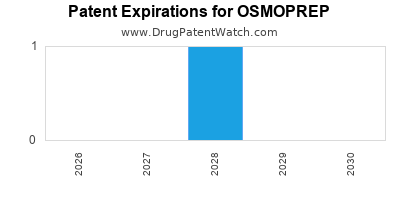
OSMOPREP is protected by one US patents.
Patents protecting OSMOPREP
Colonic purgative composition with soluble binding agent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix Pharms | OSMOPREP | sodium phosphate, dibasic, anhydrous; sodium phosphate, monobasic, monohydrate | TABLET;ORAL | 021892-001 | Mar 16, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for OSMOPREP
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Salix Pharms | OSMOPREP | sodium phosphate, dibasic, anhydrous; sodium phosphate, monobasic, monohydrate | TABLET;ORAL | 021892-001 | Mar 16, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for OSMOPREP
See the table below for patents covering OSMOPREP around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2308476 | Composition purgative pour le colon à agent de liaison soluble (Colonic purgative composition with soluble binding agent) | ⤷ Try a Trial |
| Spain | 2584866 | ⤷ Try a Trial | |
| South Korea | 101268559 | ⤷ Try a Trial | |
| Brazil | PI0416702 | FORMULAÇÃO PURGATIVA COLÔNICA, KIT, MÉTODOS DE PURGAR O CÓLON DE UM PACIENTE, DE TRATAR UM PACIENTE COM, OU SUSCETÍVEL A, UM DISTÚRBIO GASTROINTESTINAL, E DE TRATAR UM PACIENTE SOFRENDO DE, OU SUSCETÍVEL À, CONSTIPAÇÃO, E, PROCESSO DE PRODUZIR A FORMULAÇÃO PURGATIVA COLÔNICA | ⤷ Try a Trial |
| Austria | 235894 | ⤷ Try a Trial | |
| Australia | 2011202346 | Colonic purgative composition with soluble binding agent | ⤷ Try a Trial |
| European Patent Office | 0858326 | PREPARATIONS PURGATIVES NON AQUEUSES POUR LE COLON (NON-AQUEOUS COLONIC PURGATIVE FORMULATIONS) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OSMOPREP
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0268956 | SPC/GB98/040 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: RABEPRAZOLE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE SODIUM SALT; REGISTERED: UK 10555/0010 19980508; UK 10555/0008 19980508 |
| 2673237 | LUC00111 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SODIUM ZIRCONIUM CYCLOSILICATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1173 20180326 |
| 2380576 | 20C1048 | France | ⤷ Try a Trial | PRODUCT NAME: SEL DE SODIUM DE L'ACIDE DESOXYCHOLIQUE; NAT. REGISTRATION NO/DATE: NL46299 20180810; FIRST REGISTRATION: IS - IS/1/16/071/01 20160729 |
| 2822954 | 18C1035 | France | ⤷ Try a Trial | PRODUCT NAME: BICTEGRAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER BICTEGRAVIR DE SODIUM; REGISTRATION NO/DATE: EU/1/18/1289 20180625 |
| 1856135 | LUC00153 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: FOSTAMATINIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE FOSTAMATINIB, OU UN HYDRATE, SOLVATE OU N-OXYDE DE FOSTAMATINIB OU LE SEL PHARMACEUTIQUEMENT ACCEPTABLE DE FOSTAMATINIB, EN PARTICULIER FOSTAMATINIB DISODIUM, EVENTUELLEMENT SOUS FORME D'HYDRATE; AUTHORISATION NUMBER AND DATE: EU/1/19/1405 20200113 |
| 2203431 | CR 2015 00014 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150119 |
| 3141251 | 301099 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, SODIUM SULPHATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE AND THE SECOND DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, ASCORBIC ACID, SODIUM ASCORBATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE; NATIONAL REGISTRATION NO/DATE: RVG 120195 20171114; FIRST REGISTRATION: IS IS/1/17/083/01 20171016 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


