Rhythm Company Profile
✉ Email this page to a colleague
What is the competitive landscape for RHYTHM, and when can generic versions of RHYTHM drugs launch?
RHYTHM has one approved drug.
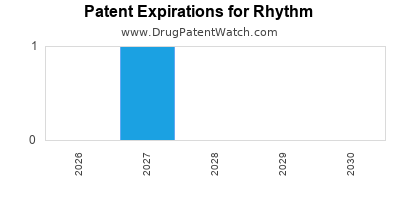
There are three US patents protecting RHYTHM drugs.
There are seventy-eight patent family members on RHYTHM drugs in twenty-one countries and seventeen supplementary protection certificates in eight countries.
Drugs and US Patents for Rhythm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | 8,039,435 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | 11,129,869 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Rhythm | IMCIVREE | setmelanotide acetate | SOLUTION;SUBCUTANEOUS | 213793-001 | Nov 25, 2020 | RX | Yes | Yes | 9,458,195 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Rhythm Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 101676298 | ⤷ Try a Trial |
| Poland | 2548568 | ⤷ Try a Trial |
| Australia | 2019200101 | ⤷ Try a Trial |
| Canada | 3044795 | ⤷ Try a Trial |
| Spain | 2604328 | ⤷ Try a Trial |
| Israel | 201732 | ⤷ Try a Trial |
| Denmark | 3354273 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Rhythm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2970389 | CA 2021 00054 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTID ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/21/1564 20210719 |
| 2970389 | 2021C/551 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTIDE, ALSMEDE DOOR HET BASISOCTROOI BESCHERMDE, THERAPEUTISCH EQUIVALENTE AFGELEIDEN ERVAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1564 20210719 |
| 2236151 | CA 2021 00053 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTID ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/21/1564 20210719 |
| 2236151 | 301150 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTIDE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/21/1564 20210719 |
| 2970389 | 21C1059 | France | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTIDE OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/21/1564 20210719 |
| 2236151 | 52/2021 | Austria | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTID; REGISTRATION NO/DATE: EU/1/21/1564 (MITTEILUNG) 20210719 |
| 2236151 | 2021C/557 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SETMELANOTIDE, ALSMEDE DOOR HET BASISOCTROOI BESCHERMDE, THERAPEUTISCH EQUIVALENTE AFGELEIDEN ERVAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1564 20210719 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.