Purdue Pharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for PURDUE PHARMA, and what generic alternatives to PURDUE PHARMA drugs are available?
PURDUE PHARMA has eleven approved drugs.
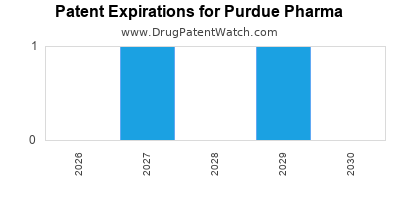
There are forty-six US patents protecting PURDUE PHARMA drugs.
There are four hundred and fifty-seven patent family members on PURDUE PHARMA drugs in fifty-one countries and eight supplementary protection certificates in five countries.
Summary for Purdue Pharma
| International Patents: | 457 |
| US Patents: | 46 |
| Tradenames: | 11 |
| Ingredients: | 11 |
| NDAs: | 11 |
| Patent Litigation for Purdue Pharma: | See patent lawsuits for Purdue Pharma |
| PTAB Cases with Purdue Pharma as petitioner: | See PTAB cases with Purdue Pharma as petitioner |
| PTAB Cases with Purdue Pharma as patent owner: | See PTAB cases with Purdue Pharma as patent owner |
Drugs and US Patents for Purdue Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-007 | Nov 20, 2014 | AB | RX | Yes | No | 9,492,389 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Purdue Pharma Lp | OXYCONTIN | oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022272-002 | Apr 5, 2010 | RX | Yes | No | 8,894,987 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Purdue Pharma Lp | ADHANSIA XR | methylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 212038-006 | Feb 27, 2019 | DISCN | Yes | No | 10,512,612 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Purdue Pharma Lp | ADHANSIA XR | methylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 212038-002 | Feb 27, 2019 | DISCN | Yes | No | 10,722,473 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-001 | Nov 20, 2014 | AB | RX | Yes | Yes | 9,775,809 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Purdue Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-002 | Nov 20, 2014 | 10,130,591 | ⤷ Try a Trial |
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-003 | Nov 20, 2014 | 8,309,060 | ⤷ Try a Trial |
| Purdue Pharma Lp | HYSINGLA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 206627-004 | Nov 20, 2014 | 8,647,667 | ⤷ Try a Trial |
| Purdue Pharma Lp | PALLADONE | hydromorphone hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021044-002 | Sep 24, 2004 | 6,335,033 | ⤷ Try a Trial |
| Purdue Pharma Lp | TARGINIQ | naloxone hydrochloride; oxycodone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205777-002 | Jul 23, 2014 | 9,555,000 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for PURDUE PHARMA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 100 mg, 200 mg and 300 mg | ➤ Subscribe | 2009-06-18 |
| ➤ Subscribe | Transdermal System | 5 mcg/hr, 10 mcg/hr, and 20 mcg/hr | ➤ Subscribe | 2013-06-06 |
| ➤ Subscribe | Extended-release Tablets | 20 mg, 60 mg, and 120 mg | ➤ Subscribe | 2015-04-15 |
| ➤ Subscribe | Extended-release Tablets | 15 mg | ➤ Subscribe | 2007-02-15 |
| ➤ Subscribe | Sublingual Tablets | 1.75 mg and 3.5 mg | ➤ Subscribe | 2012-04-10 |
| ➤ Subscribe | Transdermal System | 15 mcg/hr | ➤ Subscribe | 2013-12-16 |
| ➤ Subscribe | Extended-release Tablets | 30 mg, 40 mg, 80 mg, and 100 mg | ➤ Subscribe | 2015-05-08 |
| ➤ Subscribe | Extended-release Tablets | 30 mg and 60 mg | ➤ Subscribe | 2007-01-03 |
International Patents for Purdue Pharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2012188439 | ⤷ Try a Trial |
| Japan | 5259183 | ⤷ Try a Trial |
| Norway | 337669 | ⤷ Try a Trial |
| Canada | 2572491 | ⤷ Try a Trial |
| Denmark | 2082742 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Purdue Pharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0566709 | C300152 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TRAMADOLI HYDROCHLORIDUM EN PARACETAMOLUM; NAT. REGISTRATION NO/DATE: RVG 28113 20030115; FIRST REGISTRATION: 359 228-3 2002050405 |
| 2236132 | 122015000006 | Germany | ⤷ Try a Trial | PRODUCT NAME: ZOLPIDEM UND PHARMAZEUTISCH VERTRAEGLICHE SALZE DAVON; NAT. REGISTRATION NO/DATE: 83439.00.00 83440.00.00 20120725 FIRST REGISTRATION: BELGIEN BE424286 BE424295 20120718 |
| 2236132 | 92636 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ZOLPIDEM ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES |
| 2236132 | C300714 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ZOLPIDEM EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; NAT. REGISTRATION NO/DATE: RVG 108438 - 439 20130624; FIRST REGISTRATION: BE424286BE424295 2012180718 |
| 2236132 | CA 2015 00004 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ZOLPIDEM OG FARMACEUTISK ACCEPTABLE SALTE HERAF, HERUNDER ZOLPIDEMTARTRAT; NAT. REG. NO/DATE: 47607 OG 47608 20120719; FIRST REG. NO/DATE: (B BE424286 OG BE424295 20120718 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.