Collegium Pharm Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for COLLEGIUM PHARM INC, and what generic alternatives to COLLEGIUM PHARM INC drugs are available?
COLLEGIUM PHARM INC has four approved drugs.
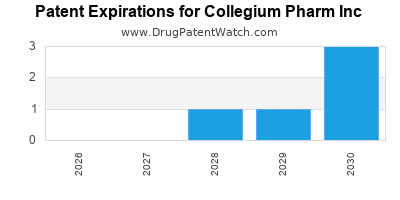
There are sixteen US patents protecting COLLEGIUM PHARM INC drugs.
There are two hundred and forty patent family members on COLLEGIUM PHARM INC drugs in thirty-one countries and twelve supplementary protection certificates in eleven countries.
Summary for Collegium Pharm Inc
| International Patents: | 240 |
| US Patents: | 16 |
| Tradenames: | 3 |
| Ingredients: | 2 |
| NDAs: | 4 |
Drugs and US Patents for Collegium Pharm Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collegium Pharm Inc | XTAMPZA ER | oxycodone | CAPSULE, EXTENDED RELEASE;ORAL | 208090-005 | Apr 26, 2016 | RX | Yes | Yes | 9,968,598 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Collegium Pharm Inc | NUCYNTA ER | tapentadol hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 200533-002 | Aug 25, 2011 | RX | Yes | No | 11,344,512 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Collegium Pharm Inc | XTAMPZA ER | oxycodone | CAPSULE, EXTENDED RELEASE;ORAL | 208090-003 | Apr 26, 2016 | RX | Yes | No | 9,737,530 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Collegium Pharm Inc | NUCYNTA | tapentadol hydrochloride | TABLET;ORAL | 022304-001 | Nov 20, 2008 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Collegium Pharm Inc | NUCYNTA ER | tapentadol hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 200533-003 | Aug 25, 2011 | RX | Yes | No | 11,344,512 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Collegium Pharm Inc | XTAMPZA ER | oxycodone | CAPSULE, EXTENDED RELEASE;ORAL | 208090-005 | Apr 26, 2016 | RX | Yes | Yes | 10,188,644 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Collegium Pharm Inc | XTAMPZA ER | oxycodone | CAPSULE, EXTENDED RELEASE;ORAL | 208090-004 | Apr 26, 2016 | RX | Yes | No | 10,004,729 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Collegium Pharm Inc
Paragraph IV (Patent) Challenges for COLLEGIUM PHARM INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | x50 mg, 75 mg, and 100 mg | ➤ Subscribe | 2012-11-20 |
| ➤ Subscribe | Oral Solution | 20 mg/mL | ➤ Subscribe | 2013-12-20 |
| ➤ Subscribe | Oral Solution | 20 mg/mL | ➤ Subscribe | 2013-12-30 |
| ➤ Subscribe | Extended-release Tablets | 50 mg, 100 mg, 150 mg, 200 mg, and 250 mg | ➤ Subscribe | 2012-11-20 |
International Patents for Collegium Pharm Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Taiwan | I381860 | ⤷ Try a Trial |
| European Patent Office | 1740156 | ⤷ Try a Trial |
| Peru | 20050814 | ⤷ Try a Trial |
| European Patent Office | 1842533 | ⤷ Try a Trial |
| European Patent Office | 2422778 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005123039 | ⤷ Try a Trial |
| Denmark | 2478896 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Collegium Pharm Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1685839 | 92292 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON D OXYCODONE EN TANT QUE COMPOSANT A ET DE NALOXONE EN TANT QUE COMPOSANT B SOUS TOUTES LES FORMES PROTEGES PAR LE BREVET DE BASE |
| 1439829 | C 2011 002 | Romania | ⤷ Try a Trial | PRODUCT NAME: TAPENTADOL SAU O SARE ACCEPTABILA FARMACEUTIC AACESTUIA; NATIONAL AUTHORISATION NUMBER: RO 3279/2011/01 - RO 3279/2011/22; DATE OF NATIONAL AUTHORISATION: 20110228; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): DE 75.046; DATE OF FIRST AUTHORISATION IN EEA: 20100819 |
| 0693475 | 12C0016 | France | ⤷ Try a Trial | PRODUCT NAME: TAPENTADOL SOUS FORME DE SA BASE LIBRE OU SOUS FORME D'UN DE SES SELS D'ACIDES ACCEPTABLES DU POINT DE VUE PHYSIOLOGIQUE; NAT. REGISTRATION NO/DATE: NL40884 20111003; FIRST REGISTRATION: DE - 76261.00.00 20100819 |
| 0693475 | C300541 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TAPENTADOL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, IN HET BIJZONDER TAPENTADOL HYDROCHLORIDE; NAT. REGISTRATION NO/DATE: RVG 110721-110724 RVG 110728-110731 20120316; FIRST REGISTRATION: DE 75043.00.00 - 75045.00.00, 75046.00.00, 76261.00.00 - 76270.00.00 20120819 |
| 1439829 | 99 1-2011 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: TAPENTADOL; NAT. REGISTRATION NO/DATE: 65/0667-0674/10-S, 65/090-0697/10-S 20101012; FIRST REGISTRATION: DE 75043-75048.00.00, 76261-76270.00.00 20100819 |
| 0693475 | SPC/GB11/031 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TAPENTADOL IN THE FORM OF ITS BASE OR A SALT OF A PHYSIOLOGICALLY COMPATIBLE ACID, ESPECIALLY THE HYDROCHLORIDE SALT.; REGISTERED: DE 75043 20100819; UK PL 21727/0032 - 0050 20110204 |
| 0693475 | 2011/010 | Ireland | ⤷ Try a Trial | PRODUCT NAME: TAPENTADOL IN BASIC FORM OR IN THE FORM OF A SALT OF A PHYSIOLOGICALLY COMPATIBLE ACID, ESPECIALLY TAPENTADOL HYDROCHLORIDE; NAT REGISTRATION NO/DATE: PA1189/007/001-008 20101221; FIRST REGISTRATION NO/DATE: PA1189/008/001-008 21/12/2010 GERMANY 75043.00.00 75044.00.00 75045.00.00 19/08/2010 GERMANY 76261.00.00 76262.00.00 76263.00.00 76264.00.00 76265.00.00 19/08/2010 GERMANY 75046.00.00 75047.00.00 75048.00.00 19/08/2010 GERMANY 76266.00.00 76267.00.00 76268.00.0076269.00.00 76270.00.00 20100819 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.