Amneal Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AMNEAL, and when can generic versions of AMNEAL drugs launch?
AMNEAL has three hundred and eighty-seven approved drugs.
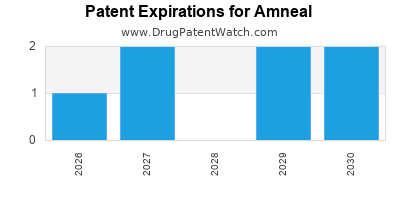
There are six US patents protecting AMNEAL drugs. There are seven tentative approvals on AMNEAL drugs.
There are sixteen patent family members on AMNEAL drugs in twelve countries and eight hundred supplementary protection certificates in eighteen countries.
Summary for Amneal
| International Patents: | 16 |
| US Patents: | 6 |
| Tradenames: | 293 |
| Ingredients: | 285 |
| NDAs: | 387 |
| Patent Litigation for Amneal: | See patent lawsuits for Amneal |
| PTAB Cases with Amneal as petitioner: | See PTAB cases with Amneal as petitioner |
Drugs and US Patents for Amneal
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amneal | AZATHIOPRINE | azathioprine | TABLET;ORAL | 074069-003 | Nov 2, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms Ny | CETIRIZINE HYDROCHLORIDE HIVES RELIEF | cetirizine hydrochloride | TABLET;ORAL | 078780-003 | Jan 21, 2010 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Amneal | LEVOTHYROXINE SODIUM | levothyroxine sodium | TABLET;ORAL | 210831-012 | Feb 19, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Amneal | CARBOPROST TROMETHAMINE | carboprost tromethamine | INJECTABLE;INJECTION | 215337-001 | Jan 27, 2022 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Amneal
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 5,466,699*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 6,750,237*PED | ⤷ Try a Trial |
| Amneal | ACTIVELLA | estradiol; norethindrone acetate | TABLET;ORAL | 020907-001 | Nov 18, 1998 | RE36247 | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 6,750,237*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for AMNEAL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Nasal Spray | 2.5 mg/spray | ➤ Subscribe | 2016-06-09 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Nasal Spray | 5 mg/spray | ➤ Subscribe | 2013-11-14 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
International Patents for Amneal Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Spain | 2795421 | ⤷ Try a Trial |
| European Patent Office | 4003323 | ⤷ Try a Trial |
| Mexico | 2022001139 | ⤷ Try a Trial |
| Canada | 3148812 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Amneal Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0193926 | 98C0038 | Belgium | ⤷ Try a Trial | PRODUCT NAME: RIVASTIGMINE; NAT. REGISTRATION NO/DATE: EU/1/98/066/001 19980512; FIRST REGISTRATION: CH 54275 01 19970731 |
| 2139494 | 122020000043 | Germany | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN UND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160715 |
| 2203431 | CA 2015 00014 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DASABUVIR OR A SALT THEREOF, INCLUDING DASABUVIR SODIUM MONOHYDRATE; REG. NO/DATE: EU/1/14/983 20150115 |
| 0443983 | C300445 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: VALSARTAN, AMLODIPINE EN HYDROCHLOORTHIAZIDE EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; REGISTRATION NO/DATE: EU/1/09/569/001-060 20091016 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.