WAKIX Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Wakix, and what generic alternatives are available?
Wakix is a drug marketed by Harmony and is included in one NDA. There are three patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-one patent family members in thirty-one countries.
The generic ingredient in WAKIX is pitolisant hydrochloride. One supplier is listed for this compound. Additional details are available on the pitolisant hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Wakix
Wakix was eligible for patent challenges on August 14, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 7, 2030. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for WAKIX
| International Patents: | 61 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 64 |
| Patent Applications: | 113 |
| Drug Prices: | Drug price information for WAKIX |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for WAKIX |
| What excipients (inactive ingredients) are in WAKIX? | WAKIX excipients list |
| DailyMed Link: | WAKIX at DailyMed |
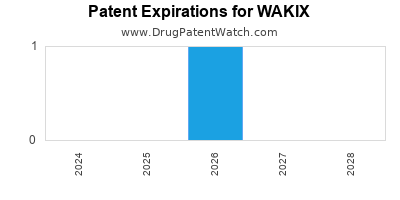

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for WAKIX
Generic Entry Date for WAKIX*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Anatomical Therapeutic Chemical (ATC) Classes for WAKIX
Paragraph IV (Patent) Challenges for WAKIX
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| WAKIX | Tablets | pitolisant hydrochloride | 4.45 mg and 17.8 mg | 211150 | 7 | 2023-08-14 |
US Patents and Regulatory Information for WAKIX
WAKIX is protected by five US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of WAKIX is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting WAKIX
Monohydrochloride salt of 1-[3-[3-(4-chlorophenyl) propoxy] propyl] -piperidine
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Monohydrochloride salt of 1-[3-[3-(4-chlorophenyl) propoxy]propyl]-piperidine
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING EXCESSIVE DAYTIME SLEEPINESS IN PATIENTS WITH NARCOLEPSY
Monohydrochloride salt of 1-[3-[3-(4-chlorophenyl) propoxy]propyl]-piperidine
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING CATAPLEXY IN PATIENTS WITH NARCOLEPSY
Treatment of Parkinson's disease, obstructive sleep apnea, dementia with Lewy bodies, vascular dementia with non-imidazole alkylamines histamine H.sub.3-receptor ligands
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING EXCESSIVE DAYTIME SLEEPINESS IN PATIENTS WITH NARCOLEPSY
Treatment of Parkinson's disease, obstructive sleep apnea, dementia with Lewy bodies, vascular dementia with non-imidazole alkylamines histamine H.sub.3-receptor ligands
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING CATAPLEXY IN PATIENTS WITH NARCOLEPSY
FDA Regulatory Exclusivity protecting WAKIX
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE TREATMENT OF EXCESSIVE DAYTIME SLEEPINESS (EDS) IN ADULT PATIENTS WITH NARCOLEPSY
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF CATAPLEXY IN ADULT PATIENTS WITH NACROLEPSY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for WAKIX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for WAKIX
When does loss-of-exclusivity occur for WAKIX?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4734
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 91716
Estimated Expiration: ⤷ Try a Trial
Patent: 02154
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 97016
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1155793
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0080446
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 08428
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 46384
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 90858
Estimated Expiration: ⤷ Try a Trial
Patent: 46384
Estimated Expiration: ⤷ Try a Trial
Germany
Patent: 2005005941
Estimated Expiration: ⤷ Try a Trial
Patent: 2006001938
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 72604
Estimated Expiration: ⤷ Try a Trial
Patent: 08530050
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07009574
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 103
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 46384
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 46384
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 624
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 46384
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1344271
Estimated Expiration: ⤷ Try a Trial
Patent: 070101381
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 09948
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 93711
Estimated Expiration: ⤷ Try a Trial
Patent: 0639157
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 369
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering WAKIX around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2008534572 | ⤷ Try a Trial | |
| Eurasian Patent Organization | 200702135 | ЛЕЧЕНИЕ БОЛЕЗНИ ПАРКИНСОНА, ОБСТРУКТИВНОГО СИНДРОМА АПНОЭ ВО СНЕ, СЛАБОУМИЯ С ТЕЛЬЦАМИ ЛЬЮИ, СОСУДИСТОЙ ДЕМЕНЦИИ С ПОМОЩЬЮ НЕ СОДЕРЖАЩИХ ИМИДАЗОЛА АЛКИЛАМИНОВЫХ ЛИГАНДОВ ГИСТАМИНОВЫХ Н3-РЕЦЕПТОРОВ | ⤷ Try a Trial |
| Croatia | P20130748 | ⤷ Try a Trial | |
| New Zealand | 561940 | Treatment of parkinson's disease, obstructive sleep apnea, dementia with lewy bodies, vascular dementia with non-imidazole alkylamines histamine H3-receptor ligands | ⤷ Try a Trial |
| Canada | 2597016 | SEL DE MONOHYDROCHLORURE DE 1-[3-[3-(4-CHLOROPHENYL)PROPOXY]PROPYL]-PIPERIDINE (MONOHYDROCHLORIDE SALT OF 1- [3- [3- (4-CHLOROPHENYL) PROPOXY] PROPYL] -PIPERIDINE) | ⤷ Try a Trial |
| Japan | 2016106142 | 非イミダゾールアルキルアミンヒスタミンH3−受容体リガンドによるパーキンソン病、閉塞性睡眠時無呼吸、レビー小体型認知症、血管性認知症の治療 (TREATMENT OF PARKINSON'S DISEASE, OBSTRUCTIVE SLEEP APNEA, DEMENTIA WITH LEWY BODIES, VASCULAR DEMENTIA WITH NON-IMIDAZOLE ALKYLAMINES HISTAMINE H3-RECEPTOR LIGANDS) | ⤷ Try a Trial |
| South Korea | 20070101381 | MONOHYDROCHLORIDE SALT OF 1-[3-[3-(4-CHLOROPHENYL)PROPOXY]PROPYL]-PIPERIDINE | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for WAKIX
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1428820 | 16C0038 | France | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES TELS QUE LE CHLORHYDRATE; REGISTRATION NO/DATE: EU/1/15/1068 20160331 |
| 1428820 | 499 | Finland | ⤷ Try a Trial | |
| 1428820 | CA 2016 00042 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT OG FARMACEUTISK ACCEPTABLE SALTE HERAF, SASOM PITOLISANT HYDROCHLORID; REG. NO/DATE: EU/1/15/1068 20160404 |
| 1428820 | 40/2016 | Austria | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT UND SEINE PHARMAZEUTISCH VERTRAEGLICHEN SALZE EINSCHLIESSLICH DEM HYDROCHLORID; REGISTRATION NO/DATE: EU/1/15/1068 (MITTEILUNG) 20160404 |
| 1428820 | CR 2016 00042 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT OG FARMACEUTISK ACCEPTABLE SALTE HERAF, SASOM PITOLISANT HYDROCHLORID; REG. NO/DATE: EU/1/15/1068 20160404 |
| 1428820 | 122016000073 | Germany | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT UND DESSEN PHARMAZEUTISCH VERTRAEGLICHE SALZE, WIE DAS HYDROCHLORID; REGISTRATION NO/DATE: EU/1/15/1068 20160331 |
| 1428820 | 93229 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ALKYLAMINES SANS IMIDAZOLES COMME LIGANDS DE RECEPTEUR H-3 D'HISTAMINE ET LEURS APPLICATIONS THERAPEUTIQUES, FIRST REGISTRATION DATE: 20160404 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


