VOCABRIA Drug Patent Profile
✉ Email this page to a colleague
When do Vocabria patents expire, and what generic alternatives are available?
Vocabria is a drug marketed by Viiv Hlthcare and is included in one NDA. There are two patents protecting this drug.
This drug has one hundred and twenty-five patent family members in thirty-three countries.
The generic ingredient in VOCABRIA is cabotegravir sodium. One supplier is listed for this compound. Additional details are available on the cabotegravir sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Vocabria
Vocabria will be eligible for patent challenges on January 21, 2025. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 28, 2026. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for VOCABRIA
| International Patents: | 125 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 20 |
| Patent Applications: | 4 |
| What excipients (inactive ingredients) are in VOCABRIA? | VOCABRIA excipients list |
| DailyMed Link: | VOCABRIA at DailyMed |
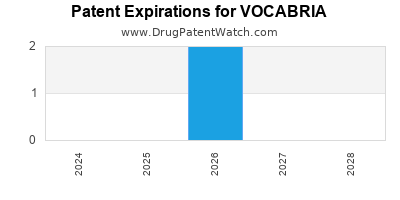
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VOCABRIA
Generic Entry Date for VOCABRIA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for VOCABRIA
Anatomical Therapeutic Chemical (ATC) Classes for VOCABRIA
US Patents and Regulatory Information for VOCABRIA
VOCABRIA is protected by three US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VOCABRIA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VOCABRIA
N-[(2,4-difluorophenyl)methyl]-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a- -hexahydro[1,3] oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide having HIV integrase inhibitory activity
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
(3S,11aR)-N-[2,4-difluorophenyl)methyl]-6-hydroxy-3-methyl-5,7-dioxo-2,3,5- ,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide useful as anti-HIV agent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 IN AN ADULT IN COMBINATION WITH RILPIVIRINE
(3S,11aR)-N-[2,4-difluorophenyl)methyl]-6-hydroxy-3-methyl-5,7-dioxo-2,3,5- ,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide useful as anti-HIV agent
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN ADULTS AND ADOLESCENTS 12 YEARS OF AGE AND OLDER AND WEIGHING AT LEAST 35 KG
FDA Regulatory Exclusivity protecting VOCABRIA
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Try a Trial
REVISION TO THE LABELING TO INCLUDE RESULTS FROM CLINICAL STUDY 207966 ATLAS-2M
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | VOCABRIA | cabotegravir sodium | TABLET;ORAL | 212887-001 | Jan 21, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Viiv Hlthcare | VOCABRIA | cabotegravir sodium | TABLET;ORAL | 212887-001 | Jan 21, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Viiv Hlthcare | VOCABRIA | cabotegravir sodium | TABLET;ORAL | 212887-001 | Jan 21, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Viiv Hlthcare | VOCABRIA | cabotegravir sodium | TABLET;ORAL | 212887-001 | Jan 21, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for VOCABRIA
When does loss-of-exclusivity occur for VOCABRIA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06239177
Estimated Expiration: ⤷ Try a Trial
Patent: 06307101
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 16026
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0610030
Estimated Expiration: ⤷ Try a Trial
Patent: 0617842
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 06282
Estimated Expiration: ⤷ Try a Trial
Patent: 26956
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1212903
Estimated Expiration: ⤷ Try a Trial
Patent: 1346376
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 15151
Estimated Expiration: ⤷ Try a Trial
Patent: 16331
Estimated Expiration: ⤷ Try a Trial
Patent: 20345
Estimated Expiration: ⤷ Try a Trial
Patent: 22052
Estimated Expiration: ⤷ Try a Trial
Patent: 24601
Estimated Expiration: ⤷ Try a Trial
Patent: 14024
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 74117
Estimated Expiration: ⤷ Try a Trial
Patent: 65580
Estimated Expiration: ⤷ Try a Trial
Patent: 45206
Estimated Expiration: ⤷ Try a Trial
Patent: 87225
Estimated Expiration: ⤷ Try a Trial
Patent: 84520
Estimated Expiration: ⤷ Try a Trial
Patent: 72281
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 4162
Estimated Expiration: ⤷ Try a Trial
Patent: 0702080
Estimated Expiration: ⤷ Try a Trial
Patent: 0801144
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 52434
Estimated Expiration: ⤷ Try a Trial
Patent: 74117
Estimated Expiration: ⤷ Try a Trial
Patent: 50212
Estimated Expiration: ⤷ Try a Trial
Patent: 65580
Estimated Expiration: ⤷ Try a Trial
Patent: 27007
Estimated Expiration: ⤷ Try a Trial
Patent: 45206
Estimated Expiration: ⤷ Try a Trial
Patent: 87225
Estimated Expiration: ⤷ Try a Trial
Patent: 87226
Estimated Expiration: ⤷ Try a Trial
Patent: 84519
Estimated Expiration: ⤷ Try a Trial
Patent: 84520
Estimated Expiration: ⤷ Try a Trial
Patent: 72281
Estimated Expiration: ⤷ Try a Trial
Finland
Patent: 0210017
Estimated Expiration: ⤷ Try a Trial
France
Patent: C0041
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 07227
Estimated Expiration: ⤷ Try a Trial
Patent: 72282
Estimated Expiration: ⤷ Try a Trial
Patent: 49742
Estimated Expiration: ⤷ Try a Trial
Patent: 51191
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 37795
Estimated Expiration: ⤷ Try a Trial
Patent: 44978
Estimated Expiration: ⤷ Try a Trial
Patent: 56603
Estimated Expiration: ⤷ Try a Trial
Patent: 400039
Estimated Expiration: ⤷ Try a Trial
Patent: 100022
Estimated Expiration: ⤷ Try a Trial
Patent: 100023
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6555
Estimated Expiration: ⤷ Try a Trial
Patent: 5206
Estimated Expiration: ⤷ Try a Trial
Patent: 5207
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 2006088173
Estimated Expiration: ⤷ Try a Trial
Patent: 2007049675
Estimated Expiration: ⤷ Try a Trial
Patent: 95353
Estimated Expiration: ⤷ Try a Trial
Patent: 31689
Estimated Expiration: ⤷ Try a Trial
Patent: 17257
Estimated Expiration: ⤷ Try a Trial
Patent: 08540343
Estimated Expiration: ⤷ Try a Trial
Patent: 09079058
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 465580
Estimated Expiration: ⤷ Try a Trial
Patent: 2021512
Estimated Expiration: ⤷ Try a Trial
Patent: 45206
Estimated Expiration: ⤷ Try a Trial
Patent: 84520
Estimated Expiration: ⤷ Try a Trial
Patent: 72281
Estimated Expiration: ⤷ Try a Trial
Luxembourg
Patent: 0210
Estimated Expiration: ⤷ Try a Trial
Patent: 446
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 2718
Estimated Expiration: ⤷ Try a Trial
Patent: 2216
Estimated Expiration: ⤷ Try a Trial
Patent: 07013351
Estimated Expiration: ⤷ Try a Trial
Patent: 08005137
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 460
Estimated Expiration: ⤷ Try a Trial
Patent: 879
Estimated Expiration: ⤷ Try a Trial
Netherlands
Patent: 0676
Estimated Expiration: ⤷ Try a Trial
Patent: 1109
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 2339
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 9525
Estimated Expiration: ⤷ Try a Trial
Patent: 0111
Estimated Expiration: ⤷ Try a Trial
Patent: 17010
Estimated Expiration: ⤷ Try a Trial
Patent: 21018
Estimated Expiration: ⤷ Try a Trial
Patent: 23042
Estimated Expiration: ⤷ Try a Trial
Patent: 075165
Estimated Expiration: ⤷ Try a Trial
Patent: 081892
Estimated Expiration: ⤷ Try a Trial
Patent: 161315
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 007502373
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 74117
Estimated Expiration: ⤷ Try a Trial
Patent: 65580
Estimated Expiration: ⤷ Try a Trial
Patent: 45206
Estimated Expiration: ⤷ Try a Trial
Patent: 84520
Estimated Expiration: ⤷ Try a Trial
Patent: 72281
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 74117
Estimated Expiration: ⤷ Try a Trial
Patent: 65580
Estimated Expiration: ⤷ Try a Trial
Patent: 45206
Estimated Expiration: ⤷ Try a Trial
Patent: 84520
Estimated Expiration: ⤷ Try a Trial
Patent: 72281
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 74117
Estimated Expiration: ⤷ Try a Trial
Patent: 65580
Estimated Expiration: ⤷ Try a Trial
Patent: 45206
Estimated Expiration: ⤷ Try a Trial
Patent: 87225
Estimated Expiration: ⤷ Try a Trial
Patent: 84520
Estimated Expiration: ⤷ Try a Trial
Patent: 72281
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0708970
Estimated Expiration: ⤷ Try a Trial
Patent: 0803423
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1363875
Estimated Expiration: ⤷ Try a Trial
Patent: 1504998
Estimated Expiration: ⤷ Try a Trial
Patent: 1580310
Estimated Expiration: ⤷ Try a Trial
Patent: 1848819
Estimated Expiration: ⤷ Try a Trial
Patent: 080009733
Estimated Expiration: ⤷ Try a Trial
Patent: 080064182
Estimated Expiration: ⤷ Try a Trial
Patent: 130133061
Estimated Expiration: ⤷ Try a Trial
Patent: 140097438
Estimated Expiration: ⤷ Try a Trial
Patent: 160003889
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 37268
Estimated Expiration: ⤷ Try a Trial
Patent: 46324
Estimated Expiration: ⤷ Try a Trial
Patent: 67197
Estimated Expiration: ⤷ Try a Trial
Patent: 69357
Estimated Expiration: ⤷ Try a Trial
Patent: 67868
Estimated Expiration: ⤷ Try a Trial
Patent: 43531
Estimated Expiration: ⤷ Try a Trial
Patent: 92304
Estimated Expiration: ⤷ Try a Trial
Patent: 06792
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 78931
Estimated Expiration: ⤷ Try a Trial
Patent: 0716635
Estimated Expiration: ⤷ Try a Trial
Patent: 0800988
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 568
Estimated Expiration: ⤷ Try a Trial
Viet Nam
Patent: 404
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VOCABRIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| France | 14C0041 | ⤷ Try a Trial | |
| European Patent Office | 3284519 | DÉRIVÉS DE CARBAMOYLPYRIDONE POLYCYCLIQUE DOTÉS D'UNE ACTIVITÉ INHIBITRICE DE L'INTÉGRASE DU VIH (POLYCYCLIC CARBAMOYLPYRIDONE DERIVATIVE HAVING HIV INTEGRASE INHIBITORY ACTIVITY) | ⤷ Try a Trial |
| Hungary | E037795 | ⤷ Try a Trial | |
| Eurasian Patent Organization | 200801144 | ПОЛИЦИКЛИЧЕСКОЕ КАРБАМОИЛПИРИДОНОВОЕ ПРОИЗВОДНОЕ, ОБЛАДАЮЩЕЕ ИНГИБИТОРНОЙ АКТИВНОСТЬЮ В ОТНОШЕНИИ ИНТЕГРАЗЫ ВИЧ | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VOCABRIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2465580 | C02465580/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: CABOTEGRAVIR; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67740 08.10.2021 |
| 1874117 | C300676 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DOLUTEGRAVIR OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF SOLVAAT DAARVAN, MET INBEGRIP VAN DOLUTEGRAVIR NATRIUM; REGISTRATION NO/DATE: EU/1/13/892 20140121 |
| 1874117 | C01874117/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DOLUTEGRAVIR; REGISTRATION NO/DATE: SWISSMEDIC 63052 08.05.2014 |
| 2465580 | 301109 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: CABOTEGRAVIR; REGISTRATION NO/DATE: EU/1/20/1481 20201217 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


