EMPAVELI Drug Patent Profile
✉ Email this page to a colleague
When do Empaveli patents expire, and when can generic versions of Empaveli launch?
Empaveli is a drug marketed by Apellis Pharms and is included in one NDA. There are ten patents protecting this drug.
This drug has one hundred and forty-two patent family members in twenty-five countries.
The generic ingredient in EMPAVELI is pegcetacoplan. One supplier is listed for this compound. Additional details are available on the pegcetacoplan profile page.
DrugPatentWatch® Generic Entry Outlook for Empaveli
Empaveli will be eligible for patent challenges on May 14, 2025. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 9, 2038. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for EMPAVELI
| International Patents: | 142 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Drug Prices: | Drug price information for EMPAVELI |
| What excipients (inactive ingredients) are in EMPAVELI? | EMPAVELI excipients list |
| DailyMed Link: | EMPAVELI at DailyMed |
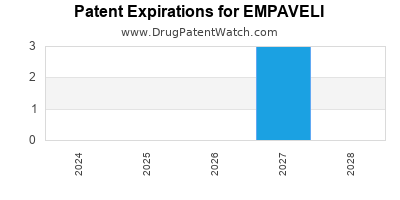

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for EMPAVELI
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for EMPAVELI
| Drug Class | Complement Inhibitor |
| Mechanism of Action | Complement Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for EMPAVELI
US Patents and Regulatory Information for EMPAVELI
EMPAVELI is protected by fifteen US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of EMPAVELI is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting EMPAVELI
Cell-reactive, long-acting, or targeted compstatin analogs and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Cell-reactive, long-acting, or targeted compstatin analogs and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Cell-reactive, long-acting, or targeted compstatin analogs and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF PEGCETACOPLAN
Dosing regimens and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF 1080 MG OF PEGCETACOPLAN
Dosing regimens and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF 1080 MG OF PEGCETACOPLAN EVERY THREE DAYS
Dosing regimens and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF 1080 MG OF PEGCETACOPLAN TWICE WEEKLY
Cell-reactive, long-acting, or targeted compstatin analogs and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF PEGCETACOPLAN
Cell-reactive, long-acting, or targeted compstatin analogs and related compositions and methods
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF PEGCETACOPLAN SO AS TO REDUCE THE SENSITIVITY OF CELLS TO COMPLEMENT-DEPENDENT DAMAGE
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF PEGCETACOPLAN
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF 1080 MG OF PEGCETACOPLAN
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF 1080 MG OF PEGCETACOPLAN EVERY THREE DAYS
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF 1080 MG OF PEGCETACOPLAN TWICE WEEKLY
Potent compstatin analogs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compstatin analogs with improved activity
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Potent compstatin analogs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) BY ADMINISTRATION OF COMPLEMENT INHIBITOR PEGCETACOPLAN
FDA Regulatory Exclusivity protecting EMPAVELI
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF ADULT PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH)
Exclusivity Expiration: ⤷ Sign Up
INFORMATION ADDED TO THE LABELING TO DESCRIBE THE RESULTS OF STUDY APL2-308
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apellis Pharms | EMPAVELI | pegcetacoplan | SOLUTION;SUBCUTANEOUS | 215014-001 | May 14, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Apellis Pharms | EMPAVELI | pegcetacoplan | SOLUTION;SUBCUTANEOUS | 215014-001 | May 14, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Apellis Pharms | EMPAVELI | pegcetacoplan | SOLUTION;SUBCUTANEOUS | 215014-001 | May 14, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Apellis Pharms | EMPAVELI | pegcetacoplan | SOLUTION;SUBCUTANEOUS | 215014-001 | May 14, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Apellis Pharms | EMPAVELI | pegcetacoplan | SOLUTION;SUBCUTANEOUS | 215014-001 | May 14, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for EMPAVELI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Swedish Orphan Biovitrum AB (publ) | Aspaveli | pegcetacoplan | EMEA/H/C/005553 Aspaveli is indicated in the treatment of adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who are anaemic after treatment with a C5 inhibitor for at least 3 months. |
Authorised | no | no | yes | 2021-12-13 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for EMPAVELI
When does loss-of-exclusivity occur for EMPAVELI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 18249627
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2019020955
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 59304
Estimated Expiration: ⤷ Sign Up
China
Patent: 0831544
Estimated Expiration: ⤷ Sign Up
Patent: 6059313
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 06465
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 9844
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 20516607
Estimated Expiration: ⤷ Sign Up
Patent: 23100641
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 19012033
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 19131869
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 190139931
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EMPAVELI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2023100641 | 投与レジメンならびに関連組成物および方法 (DOSING REGIMENS, AND RELATED COMPOSITIONS AND METHODS) | ⤷ Sign Up |
| Australia | 2006318333 | Potent compstatin analogs | ⤷ Sign Up |
| Spain | 2390828 | ⤷ Sign Up | |
| Cyprus | 1113441 | ⤷ Sign Up | |
| Slovenia | 3660033 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EMPAVELI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3660033 | 2290021-1 | Sweden | ⤷ Sign Up | PRODUCT NAME: PEGCETAKOPLAN; REG. NO/DATE: EU/1/21/1595 20211214 |
| 3660033 | 2022C/522 | Belgium | ⤷ Sign Up | PRODUCT NAME: PEGCETACOPLAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1595 20211214 |
| 3660033 | 301178 | Netherlands | ⤷ Sign Up | PRODUCT NAME: PEGCETACOPLAN; REGISTRATION NO/DATE: EU/1/21/1595 20211214 |
| 3660033 | 2022017 | Norway | ⤷ Sign Up | PRODUCT NAME: PEGCETACOPLAN; REG. NO/DATE: EU/1/21/1595 20211216 |
| 3660033 | 122022000034 | Germany | ⤷ Sign Up | PRODUCT NAME: PEGCETACOPLAN; REGISTRATION NO/DATE: EU/1/21/1595 20211213 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


