BYDUREON PEN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Bydureon Pen, and what generic alternatives are available?
Bydureon Pen is a drug marketed by Astrazeneca Ab and is included in one NDA. There are twenty patents protecting this drug.
This drug has four hundred and eight patent family members in forty-eight countries.
The generic ingredient in BYDUREON PEN is exenatide synthetic. There are seven drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the exenatide synthetic profile page.
DrugPatentWatch® Generic Entry Outlook for Bydureon Pen
Indicators of Generic Entry
Summary for BYDUREON PEN
| International Patents: | 408 |
| US Patents: | 20 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 26 |
| Clinical Trials: | 39 |
| Patent Applications: | 7 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for BYDUREON PEN |
| DailyMed Link: | BYDUREON PEN at DailyMed |

Recent Clinical Trials for BYDUREON PEN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The University of Texas Health Science Center, Houston | Phase 2 |
| University of Washington | Phase 3 |
| Dasman Diabetes Institute | Phase 4 |
Anatomical Therapeutic Chemical (ATC) Classes for BYDUREON PEN
US Patents and Regulatory Information for BYDUREON PEN
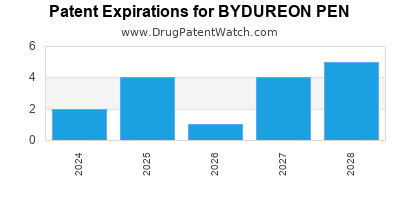
BYDUREON PEN is protected by twenty US patents and two FDA Regulatory Exclusivities.
Patents protecting BYDUREON PEN
C-aryl glucoside SGLT2 inhibitors and method
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Polymer-based sustained release device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Polymer-based sustained release device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Polymer-based sustained release device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Administering apparatus with functional drive element
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for treating diabetes and reducing body weight
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Polymer-based sustained release device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Device for administering fluid from a multi-chamber ampoule in incremental steps
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Polymer-based sustained release device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Mixing device for a two-chamber ampoule
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Ampoule comprising an ampoule holder
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Administering apparatus with functional drive element
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Administering device with holding mechanism
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method for treating diabetes
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Ampoule comprising an ampoule holder
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Polymer-based sustained release device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method for administering a fluid active substance from a multi-chamber ampoule
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for treating diabetes and reducing body weight
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting BYDUREON PEN
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BYDUREON PEN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BYDUREON PEN
See the table below for patents covering BYDUREON PEN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Cyprus | 1107883 | ⤷ Sign Up | |
| Japan | 2015071636 | 糖尿病の治療用SGLT2阻害剤としてのアミノ酸を有する(1S)−1,5−アンヒドロ−1−C−(3−((フェニル)メチル)フェニル)−D−グルシトール誘導体の結晶性溶媒和物および複合体 (CRYSTALLINE SOLVATES AND COMPLEXES OF (1S)-1,5-ANHYDRO-1-C-(3-((PHENYL)METHYL)PHENYL)-D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR TREATMENT OF DIABETES) | ⤷ Sign Up |
| China | 104382880 | Poly (lactide-co-glycolide)-based sustained release microcapsules comprising a polypeptide and a sugar | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2009100549 | ⤷ Sign Up | |
| European Patent Office | 1224195 | INHIBITEURS DE SGLT2 A BASE DE GLUCOSIDE C-ARYLE (C-ARYL GLUCOSIDE SGLT2 INHIBITORS) | ⤷ Sign Up |
| European Patent Office | 2347762 | EXENDIN POUR LE TRAITEMENT DU DIABÈTE ET LA RÉDUCTION DU POIDS (EXENDIN FOR TREATING DIABETES AND REDUCING BODY WEIGHT) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BYDUREON PEN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1506211 | CR 2014 00037 | Denmark | ⤷ Sign Up | PRODUCT NAME: ET KOMBINATIONSPRODUKT AF DAPAGLIFLOZIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, HERUNDER DAPAGLIFLOZINPROPANDIOLMONOHYDRAT OG METFORMIN ELLER SALTE DERAF, HERUNDER METFORMINHYDROCHLORID; REG. NO/DATE: EU/1/13/900 20140116 |
| 2139494 | 301054 | Netherlands | ⤷ Sign Up | PRODUCT NAME: SAXAGLIPTIN AND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160719 |
| 2139494 | 122020000043 | Germany | ⤷ Sign Up | PRODUCT NAME: SAXAGLIPTIN UND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160715 |
| 1506211 | SPC/GB13/021 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: DAPAGLIFLOZIN AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU/1/12/795/001 20121114; UK EU/1/12/795/002 20121114; UK EU/1/12/795/003 20121114; UK EU/1/12/795/004 20121114; UK EU/1/12/795/005 20121114; UK EU/1/12/795/006 20121114; UK EU/1/12/795/007 20121114; UK EU/1/12/795/008 20121114; UK EU/1/12/795/009 20121114; UK EU/1/12/795/010 20121114 |
| 2139494 | SPC/GB20/028 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: SAXAGLIPTIN AND DAPAGLIFLOZIN; REGISTERED: UK EU/1/16/1108 (NI) 20160719; UK PLGB 17901/0339 20160719 |
| 1506211 | C300677 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE VAN DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN METFORMINE OF EEN FARMACEITISCH AANVAARDBAAR ZOUT DAARVAN, ZOALS BESCHERMD DOOR HET BASISOCTROOI EP 1506211B1; REGISTRATION NO/DATE: EU/1/13/900 20140121 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


