HYDROGEN PEROXIDE - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for hydrogen peroxide and what is the scope of patent protection?
Hydrogen peroxide
is the generic ingredient in one branded drug marketed by Aclaris and is included in one NDA. There are five patents protecting this compound. Additional information is available in the individual branded drug profile pages.Hydrogen peroxide has eighteen patent family members in sixteen countries.
There are four drug master file entries for hydrogen peroxide.
Summary for HYDROGEN PEROXIDE
| International Patents: | 18 |
| US Patents: | 5 |
| Tradenames: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Drug Master File Entries: | 4 |
| Raw Ingredient (Bulk) Api Vendors: | 155 |
| Clinical Trials: | 98 |
| Patent Applications: | 5,670 |
| Formulation / Manufacturing: | see details |
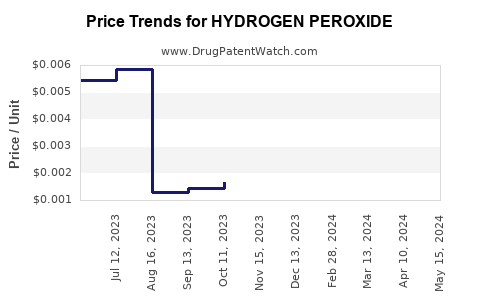
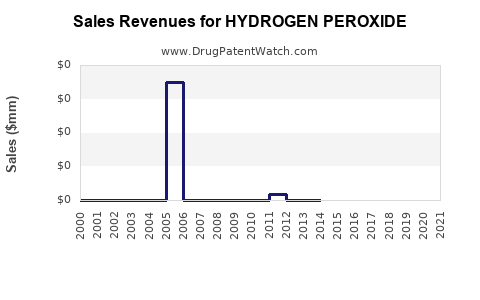
| Drug Prices: | Drug price trends for HYDROGEN PEROXIDE |
| What excipients (inactive ingredients) are in HYDROGEN PEROXIDE? | HYDROGEN PEROXIDE excipients list |
| DailyMed Link: | HYDROGEN PEROXIDE at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for HYDROGEN PEROXIDE
Generic Entry Date for HYDROGEN PEROXIDE*:
Constraining patent/regulatory exclusivity:
Dosage:
SOLUTION;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for HYDROGEN PEROXIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Louisiana State University Health Sciences Center in New Orleans | N/A |
| Synedgen, Inc. | N/A |
| University of Baghdad | Phase 3 |
Medical Subject Heading (MeSH) Categories for HYDROGEN PEROXIDE
US Patents and Regulatory Information for HYDROGEN PEROXIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for HYDROGEN PEROXIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for HYDROGEN PEROXIDE
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2015164427 | ⤷ Try a Trial | |
| Spain | 2828711 | ⤷ Try a Trial | |
| Brazil | 112016024630 | composição tópica, uso de uma composição tópica e aplicador | ⤷ Try a Trial |
| Singapore | 11201608775X | PEROXIDE FORMULATIONS AND METHODS AND APPLICATORS FOR USING THE SAME | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for HYDROGEN PEROXIDE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1968948 | CR 2021 00044 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SELUMETINIB HYDROGENSULFAT OG SOLVATER OG ANHYDRID FORMER DERAF; REG. NO/DATE: EU/1/21/1552 20210619 |
| 2523731 | 2020012 | Norway | ⤷ Try a Trial | PRODUCT NAME: OSILODROSTAT ELLER ET FARMASOEYTISK AKSEPTABELT SALT DERAV, INKLUDERT OSILODROSTAT DIHYDROGENFOSFAT; REG. NO/DATE: EU/1/19/1407 20200203 |
| 3106463 | CR 2020 00013 | Denmark | ⤷ Try a Trial | PRODUCT NAME: LAROTRECTINIB OG/ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, SAERLIGT LAROTRECTINIBSULFAT, INKLUSIV LAROTRECTINIBHYDROGENSULFAT; REG. NO/DATE: EU/1/19/1385 20190923 |
| 0281459 | SPC/GB99/001 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: CLOPIDOGREL HYDROGEN SULPHATE, THE DEXTROROTATORY ISOMER OF ALPHA-(4,5,6,7-TETRAHYDROTHIENO(3,2-C)PYRID-5-YL)(2-CHLOROPHENYL) METHYL ACETATE AS THE HYDROGEN SULPHATE SALT; REGISTERED: UK EU/1/98/069/001 19980715; UK EU/1/98/069/002 19980715; UK EU/1/98/069/003 19980715 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |