BENZOYL PEROXIDE - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for benzoyl peroxide and what is the scope of patent protection?
Benzoyl peroxide
is the generic ingredient in ten branded drugs marketed by Galderma Labs Lp, Bausch, Actavis Labs Ut Inc, Chartwell Rx, Encube, Glenmark Pharms, Mylan Pharms Inc, Padagis Israel, Taro, Zydus Pharms, Stiefel, Biofrontera, Valeant Intl, and Lyne, and is included in twenty-four NDAs. There are twenty-two patents protecting this compound. Additional information is available in the individual branded drug profile pages.Benzoyl peroxide has twenty-eight patent family members in eleven countries.
There are seven drug master file entries for benzoyl peroxide. One supplier is listed for this compound. There are two tentative approvals for this compound.
Summary for BENZOYL PEROXIDE
| International Patents: | 28 |
| US Patents: | 22 |
| Tradenames: | 10 |
| Applicants: | 14 |
| NDAs: | 24 |
| Drug Master File Entries: | 7 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 113 |
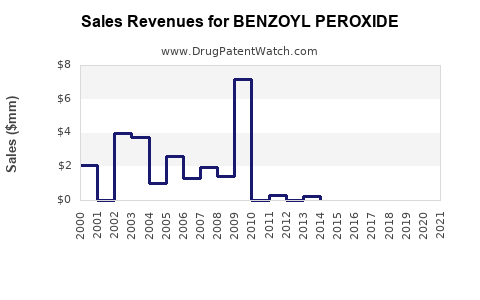
| Clinical Trials: | 107 |
| Patent Applications: | 5,591 |
| Formulation / Manufacturing: | see details |
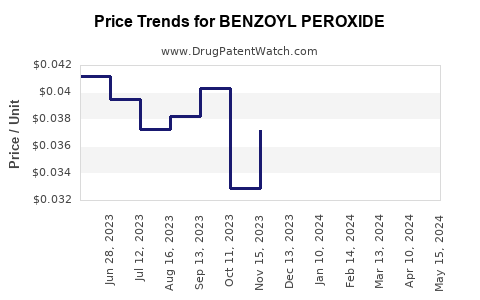
| Drug Prices: | Drug price trends for BENZOYL PEROXIDE |
| What excipients (inactive ingredients) are in BENZOYL PEROXIDE? | BENZOYL PEROXIDE excipients list |
| DailyMed Link: | BENZOYL PEROXIDE at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BENZOYL PEROXIDE
Generic Entry Date for BENZOYL PEROXIDE*:
Constraining patent/regulatory exclusivity:
Dosage:
CREAM;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BENZOYL PEROXIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| South Valley University | N/A |
| University of Missouri-Columbia | Phase 4 |
| University of Oklahoma | Phase 3 |
Generic filers with tentative approvals for BENZOYL PEROXIDE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 3.75%; EQ 1.2% BASE | GEL;TOPICAL |
| ⤷ Try a Trial | ⤷ Try a Trial | UNKNOWN | UNKNOWN |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
US Patents and Regulatory Information for BENZOYL PEROXIDE
International Patents for BENZOYL PEROXIDE
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2020170031 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 2020170030 | ⤷ Try a Trial | |
| Canada | 3130439 | METHODE DE TRAITEMENT DE LA ROSACEE CHEZ DES PATIENTS AGES DE 65 ANS ET PLUS (METHOD FOR TREATMENT OF ROSACEA IN PATIENTS AGED 65 YEARS AND OLDER) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2007015243 | ⤷ Try a Trial | |
| Brazil | PI0614143 | processo para revestir um material particulado insolével em Água, sàlido, com um àxido metÁlico, material particulado revestido, composiÇço, mÉtodo para o tratamento de uma condiÇço superficial em um induvÍduo, e, uso de material particulado revestido | ⤷ Try a Trial |
| South Africa | 200801146 | Metal oxide coating of water insoluble ingredients | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BENZOYL PEROXIDE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0526708 | C300097 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: BOSENTAN, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN HYDRAAT OF IN DE VORM VAN EEN ESTER VAN DE HYDROXYLGROEP VAN DE 2-HYDROXYETHOXY REST MET EEN ZUUR MET DE FORMULE R5-OH, WAARIN R5 EEN C1-7-ALKANOYL, BENZOYL, OF HETEROCYCLYCARBONYL VOORSTELT; NATL. REGISTRATION NO/DATE: U/1/02/220/001 - 005 20020515; FIRST REGISTRATION: CH IKS 58841 01 - 02 20020228 |
| 0137963 | 97C0042 | Belgium | ⤷ Try a Trial | PRODUCT NAME: 2-(2-BENZOYL-SUBSTITUE)-1,3-CYCLOHEXANE-DIONES; REGISTRATION NO/DATE: 8452/B 19930121 |
| 1458369 | C01458369/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: ADAPALENUM + BENZOYLIS PEROXIDUM; REGISTRATION NUMBER/DATE: SWISSMEDIC 58460 19.05.2009 |
| 1586316 | SPC/GB11/054 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC 2-AMINO-3-(4-BROMOBENZOYL)PHENYLACETIC ACID OR A PHARMACOLOGICALLY ACCEPTABLE SALT THEREOF OR A HYDRATE THEREOF; REGISTERED: UK EU/1/11/692/001 20110523 |
| 1458369 | CA 2008 00029 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ADAPALEN, BENZOYLPEROXID |
| 0186118 | SPC/GB05/029 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: MESOTRIONE (2-(4-METHYLSULPHONYL-2-NITROBENZOYL)-1,3CYCLOHEXANEDIONE); REGISTERED: AU 2726 20001016; UK 0309 OF 2005 20050218 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |