SANDOZ Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANDOZ, and what generic alternatives to SANDOZ drugs are available?
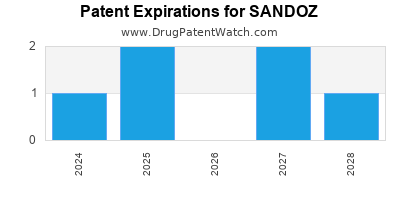
SANDOZ has four hundred and eighty-nine approved drugs.
There are nine US patents protecting SANDOZ drugs. There are thirty-three tentative approvals on SANDOZ drugs.
There are one hundred and three patent family members on SANDOZ drugs in thirty-four countries and five hundred and three supplementary protection certificates in seventeen countries.
Summary for SANDOZ
| International Patents: | 103 |
| US Patents: | 9 |
| Tradenames: | 322 |
| Ingredients: | 300 |
| NDAs: | 489 |
| Patent Litigation for SANDOZ: | See patent lawsuits for SANDOZ |
| PTAB Cases with SANDOZ as petitioner: | See PTAB cases with SANDOZ as petitioner |
Drugs and US Patents for SANDOZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandoz | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074495-001 | Dec 5, 1994 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | METOPROLOL SUCCINATE | metoprolol succinate | TABLET, EXTENDED RELEASE;ORAL | 076969-001 | Jul 31, 2006 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | IMIPRAMINE HYDROCHLORIDE | imipramine hydrochloride | TABLET;ORAL | 084936-002 | Approved Prior to Jan 1, 1982 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | METHOCARBAMOL | methocarbamol | TABLET;ORAL | 087283-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | PENICILLIN V POTASSIUM | penicillin v potassium | TABLET;ORAL | 064071-002 | Nov 30, 1995 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | LEVOFLOXACIN | levofloxacin | TABLET;ORAL | 077438-001 | Jun 20, 2011 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SANDOZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sandoz | EXELON | rivastigmine | FILM, EXTENDED RELEASE;TRANSDERMAL | 022083-001 | Jul 6, 2007 | 5,602,176 | ⤷ Try a Trial |
| Sandoz | CILOXAN | ciprofloxacin hydrochloride | OINTMENT;OPHTHALMIC | 020369-001 | Mar 30, 1998 | 4,670,444*PED | ⤷ Try a Trial |
| Sandoz | RITALIN LA | methylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021284-001 | Jun 5, 2002 | 5,837,284 | ⤷ Try a Trial |
| Sandoz | FOCALIN XR | dexmethylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021802-005 | Oct 23, 2009 | 6,635,284 | ⤷ Try a Trial |
| Sandoz | LOTREL | amlodipine besylate; benazepril hydrochloride | CAPSULE;ORAL | 020364-004 | Mar 3, 1995 | 6,162,802 | ⤷ Try a Trial |
| Sandoz | ZOFRAN | ondansetron hydrochloride | TABLET;ORAL | 020103-003 | Aug 27, 1999 | 5,344,658*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANDOZ drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release capsules | 25 mg | ➤ Subscribe | 2011-09-30 |
| ➤ Subscribe | Extended-release Tablets | 80 mg | ➤ Subscribe | 2007-03-15 |
| ➤ Subscribe | Extended-release Capsules | 10 mg | ➤ Subscribe | 2007-05-21 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2004-05-27 |
| ➤ Subscribe | Capsules | 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg and 10 mg/20 mg | ➤ Subscribe | 2004-06-09 |
| ➤ Subscribe | Ophthalmic Emulsion | 0.05% | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Oral Solution | 4 mg/5 mL | ➤ Subscribe | 2004-12-20 |
| ➤ Subscribe | Extended-release Capsules | 5mg, 10mg and 20 mg | ➤ Subscribe | 2007-03-30 |
| ➤ Subscribe | Injection | 0.05 mg/mL, 100 mL vial | ➤ Subscribe | 2008-08-29 |
| ➤ Subscribe | Extended-release Capsule | 40 mg | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Otic Suspension | 0.3%/0.1% | ➤ Subscribe | 2012-07-31 |
| ➤ Subscribe | Extended-release capsules | 35 mg | ➤ Subscribe | 2011-09-29 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2004-07-27 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2006-08-21 |
| ➤ Subscribe | Capsules | 5 mg/40 mg and 10 mg/40 mg | ➤ Subscribe | 2006-11-17 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Extended-release Capsules | 15 mg | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | For Injection | 250 mg/vial | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Extended-release Capsule | 30 mg | ➤ Subscribe | 2010-12-15 |
| ➤ Subscribe | Ophthalmic Solution | 0.00% | ➤ Subscribe | 2009-02-19 |
International Patents for SANDOZ Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 045670 | ⤷ Try a Trial |
| Taiwan | I333492 | ⤷ Try a Trial |
| Japan | 2007505861 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2008036847 | ⤷ Try a Trial |
| Mexico | 2009003042 | ⤷ Try a Trial |
| Mexico | 2009002221 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SANDOZ Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1381356 | SPC/GB14/011 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMIDE, ITS STEREOISOMER OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/13/838/001-005 20130829 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 1869025 | LUC00086 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: BREXPIPRAZOLE OU UN SEL DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/18/1294 20180730 |
| 0788360 | SPC/GB04/021 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: BORTEZOMIB OR PHARMACEUTICALLY ACCEPTABLE ESTER THEREOF, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/04/274/001 20040428 |
| 2207786 | 2023C/551 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMPOSITION COMPRENANT : DE LA CEDAZURIDINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI ; ET LA DECITABINE; AUTHORISATION NUMBER AND DATE: EU/1/23/1756 20230918 |
| 2435024 | 301102 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN FORMOTEROL (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN), GLYCOPYRROLAAT (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN) EN BUDESONIDE (MET INBEGRIP VAN DE FARMACEUTISCH AANVAARDBARE ZOUTEN, ESTERS, SOLVATEN OF ENANTIOMEREN ERVAN); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.