NOVO Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVO, and when can generic versions of NOVO drugs launch?
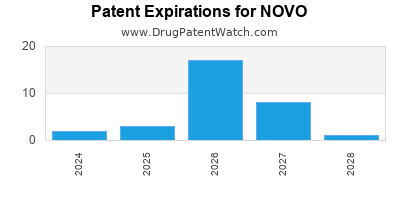
NOVO has eleven approved drugs.
There are fifty-six US patents protecting NOVO drugs.
There are four hundred and sixteen patent family members on NOVO drugs in forty countries and one hundred and eighteen supplementary protection certificates in seventeen countries.
Summary for NOVO
| International Patents: | 416 |
| US Patents: | 56 |
| Tradenames: | 11 |
| Ingredients: | 7 |
| NDAs: | 11 |
| Patent Litigation for NOVO: | See patent lawsuits for NOVO |
Drugs and US Patents for NOVO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | 8,114,833*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-004 | Oct 6, 2022 | RX | Yes | Yes | 11,446,443 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | WEGOVY | semaglutide | SOLUTION;SUBCUTANEOUS | 215256-002 | Jun 4, 2021 | RX | Yes | Yes | 9,764,003 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novo | RIVFLOZA | nedosiran sodium | SOLUTION;INJECTION | 215842-001 | Sep 29, 2023 | RX | Yes | Yes | 11,661,604 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-004 | Oct 6, 2022 | RX | Yes | Yes | 9,132,239 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-003 | Mar 28, 2022 | RX | Yes | Yes | 9,775,953 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NOVO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | 8,672,898 | ⤷ Try a Trial |
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | 6,004,297 | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | 6,899,699 | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | 6,899,699 | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | 9,486,588 | ⤷ Try a Trial |
| Novo | MACRILEN | macimorelin acetate | FOR SOLUTION;ORAL | 205598-001 | Dec 20, 2017 | 6,861,409 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 1 mg/500 mg and 2 mg/500 mg | ➤ Subscribe | 2009-04-09 |
| ➤ Subscribe | Injection | 18 mg/3 mL prefilled syringe | ➤ Subscribe | 2016-12-12 |
| ➤ Subscribe | Vaginal Tablets | 10 mcg | ➤ Subscribe | 2013-01-02 |
International Patents for NOVO Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Poland | 1863839 | ⤷ Try a Trial |
| European Patent Office | 3204497 | ⤷ Try a Trial |
| Mexico | 2014010685 | ⤷ Try a Trial |
| Serbia | 62501 | ⤷ Try a Trial |
| Brazil | 112013014942 | ⤷ Try a Trial |
| Malaysia | 195657 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for NOVO Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2209800 | 14C0085 | France | ⤷ Try a Trial | PRODUCT NAME: LIRAGLUTIDE ET INSULINE DEGLUDEC; REGISTRATION NO/DATE: EU/1/14/947 20140918 |
| 0136011 | 99C0003 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND NORETHISTERONE; FIRST REGISTRATION NO/DATE: 403 IS 106 F3 19980928; FIRST REGISTRATION: SE 14007 19980306 |
| 1214076 | SZ 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON |
| 2498758 | LUC00152 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: METFORMINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; SAXAGLIPTINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; DAPAGLIFLOZINE OU UN SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; AUTHORISATION NUMBER AND DATE: EU/1/19/1401 20191113 |
| 1412357 | PA2008013 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINUM PHOSPHAS MONOHYDRICUS, METFORMINI HYDROCHLORIDUM; REG. NO/DATE: EU/1/08/455/001-014 20080716 |
| 1214076 | 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON; REGISTRATION NO/DATE: 1-27586 20080612 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.